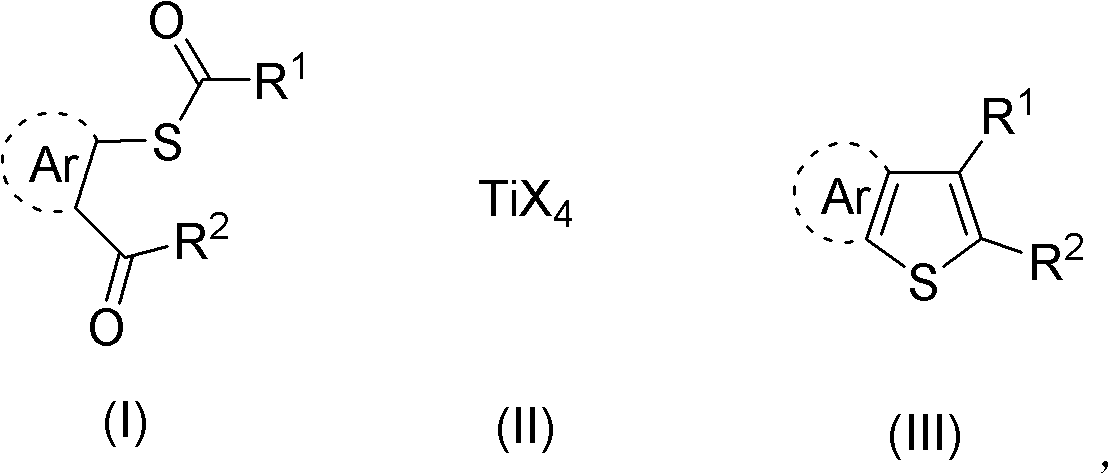
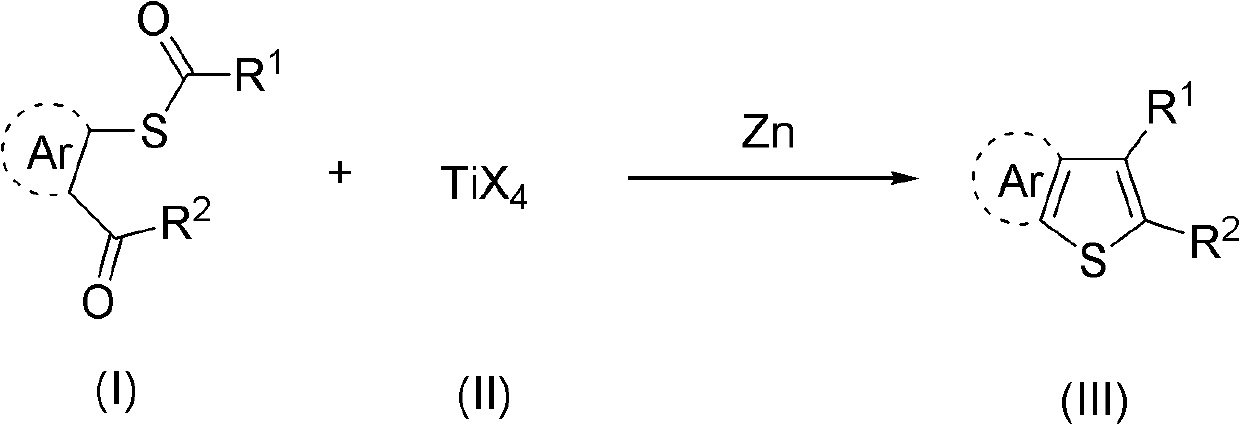
Synthesis method of aryl thiophthene derivative
A technology of thiophene derivatives and synthesis methods, which is applied in the general synthesis of aryl thiophene derivatives, in the field of aryl thiophene derivatives, can solve the problems of expensive catalysts and low functional group tolerance, and achieve simple and easy experimental equipment and operation The effect of scientific and reasonable synthetic method and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
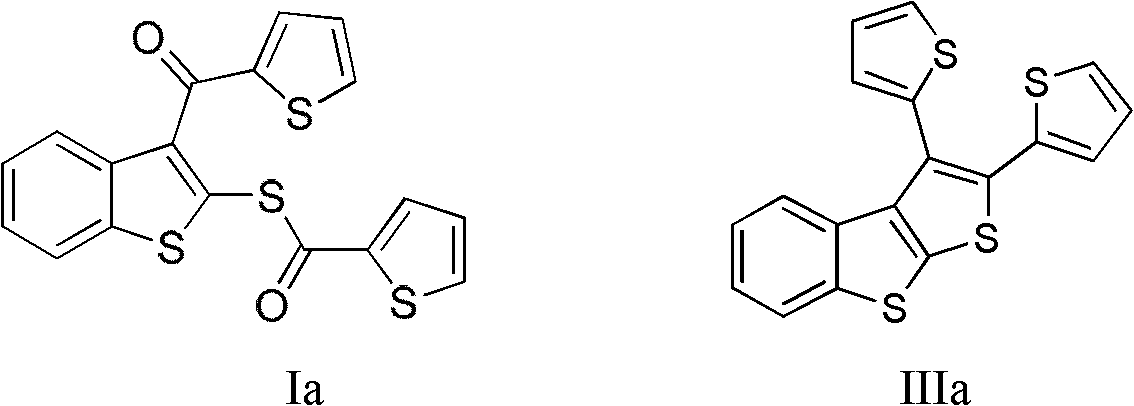
[0029] Embodiment 1——preparation of formula IIIa (R 1 =R 2 =Thienyl (Thiophenyl)) compound:
[0030]
[0031] Add 1mmol of compound Ia to a 20mL reaction tube under nitrogen protection, add 5mL of 1,4-dioxane, use an ice-water bath to keep the reaction temperature at 0°C, add 10mmol of TiCl 4 , after the addition, the temperature was naturally raised to 25°C, and the reaction was carried out with magnetic stirring for half an hour. Add 10 mmol of zinc powder, raise the temperature to 110°C, and react for nine hours. Add water to quench the reaction, extract the aqueous phase three times with dichloromethane, combine the organic phases, wash with saturated sodium chloride solution, add anhydrous magnesium sulfate to the organic phase after liquid separation, dry for 30 minutes, filter under normal pressure, and concentrate the filtrate. Decolorization and separation on a silica gel column, using dichloromethane as the eluent, yielded 0.112 g of benzothiophene derivative I...
Embodiment 2
[0032] Embodiment 2——preparation of formula IIIb (R 1 =R 2 = Propyl (Pr)) compound:
[0033]
[0034] The synthetic route is basically the same as in Example 1, and the aftertreatment is the same. The difference is that this synthesis uses 1mmol Ib as the starting material, and the TiCl used 4 and zinc powder are both 5mmol, raised to 130°C for six hours in the reaction step b) and reacted for six hours to obtain 0.095g of pure product IIIb with an isolated yield of 28%. The NMR data of this compound are as follows: 1 H NMR (300MHz, CDCl 3 , Me 4 Si, 25°C): δ=1.04-1.14 (m, 6H, CH 3 ), 1.72-1.83 (m, 4H, CH 2 ), 2.86(t, J=7.6Hz, 2H, CH 2 ), 3.10(t, J=7.9Hz, 2H, CH 2 ), 7.32-7.47(m, 2H, CH), 7.85(d, J=7.8Hz, 1H, CH), 8.19(d, J=7.8Hz, 1H, CH); 13 C NMR (75MHz, CDCl 3 , Me 4 Si, 25°C): δ = 13.69 (1 CH 3 ), 13.90 (1 CH 3 ), 24.81 (1 CH 2 ), 25.17 (1 CH 2 ), 30.86 (1 CH 2 ), 31.15 (1 CH 2 ), 122.61(1 CH), 123.33(1 CH), 123.38(1 CH), 124.44(1 CH), 130.52(1 quat.C)...
Embodiment 3
[0035] Embodiment 3——preparation of formula IIIc (R 1 = R 2 = phenyl (Ph)) compound:
[0036]
[0037] The synthetic route is basically the same as in Example 2, and the aftertreatment is the same. The difference is that this synthesis uses 1 mmol Ic as a starting material, and finally obtains 0.200 g of pure product IIIc with an isolated yield of 63%. The NMR data of this compound are as follows: 1 H NMR (300MHz, CDCl 3 , Me 4 Si, 25°C): δ=5.74-5.77 (m, 1H, CH), 6.63-7.51 (m, 13H, CH); 13 C NMR (75MHz, CDCl 3 , Me 4 Si, 25°C): δ=122.64(1 CH), 123.38(1 CH), 123.51(1 CH), 124.05(1 CH), 127.42(1 CH), 128.14(1 CH), 128.30(2 CH), 128.72(2 CH), 129.11(2 CH), 131.20(2 CH), 131.55(1 quat.C), 132.24(1 quat.C), 133.77(1 quat.C), 134.37(1 quat.C), 136.41(1 quat.C), 137.18(1 quat.C), 139.87(1 quat.C), 140.96(1 quat.C), 141.51(1 quat.C), 142.84(1 quat.C).HRMS calcd .for C 24 h 14 S 3 [M+H] + : 399.03304, found 399.03243.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com