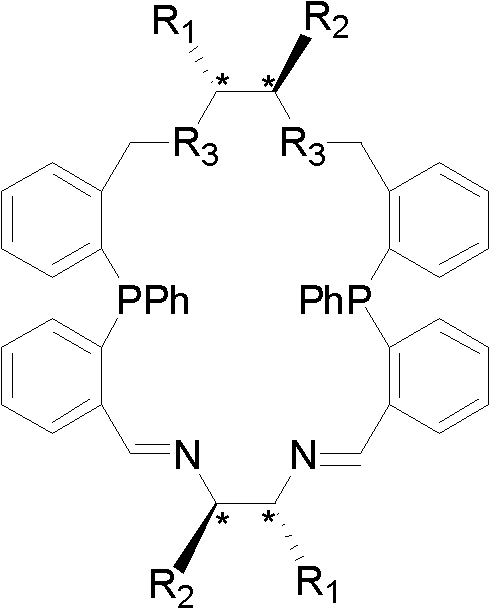
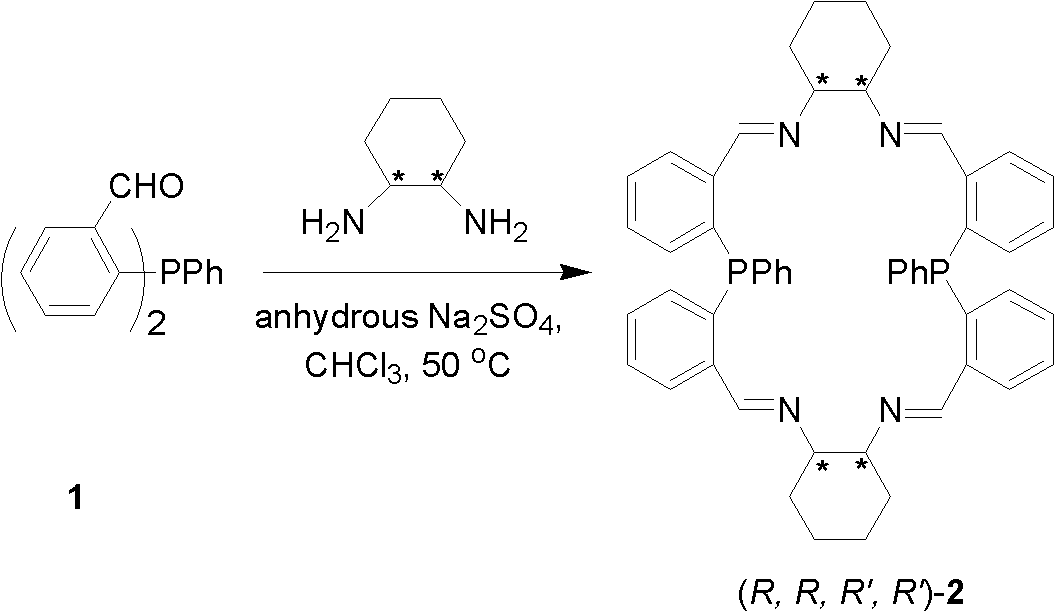Chiral macrocyclic aminophosphine ligand, and preparation method and application thereof
A technology of phosphine ligands and macrocyclic amines, which is applied in the field of chiral macrocyclic phosphine amine ligands and its preparation, can solve the problems of low optical purity, improve enantioselectivity, improve stability and rigidity, and facilitate operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Chiral cyclic P 2 N 4 Preparation of type imidophosphine ligand (compound (R, R, R', R')-2)
[0031] The chiral ring P is given by 2 N 4 The synthetic route of type imidophosphine ligand (compound (R, R, R', R')-2):
[0032]
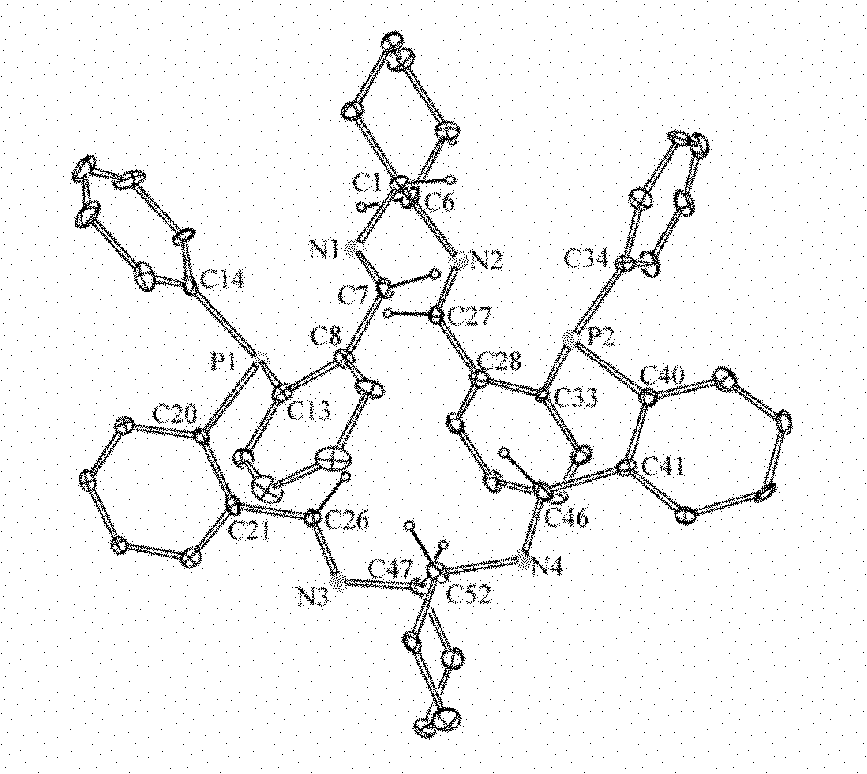
[0033] Bis(2-formylphenyl)phenylphosphine (9.55 g, 0.03 mol) and (R,R)-1,2-cyclohexanediamine (3.43 g, 0.03 mol) were added to a 400 mL round bottom flask, Chloroform (300 mL) was added under a nitrogen atmosphere, the reaction was stirred, and the temperature was rapidly raised to 40°C. After 3h, anhydrous sodium sulfate (60g) was added and reacted at 40°C for 24h. Cool to room temperature under nitrogen. Remove sodium sulfate by filtration, wash twice with saturated ammonium chloride aqueous solution (100mL×2), then dry over anhydrous sodium sulfate, filter, evaporate the solvent under reduced pressure, and recrystallize with chloroform and n-hexane to obtain yellow-green crystals (11.2 g). According to X-ray single crystal...
Embodiment 2
[0038] Example 2: Chiral cyclic P 2 (NH) 4 Preparation of Type Aminophosphine Ligand (Compound (R, R, R', R')-3)
[0039] The chiral ring P is given by 2 (NH) 4 The synthetic route of type amine phosphine ligand (compound (R, R, R', R')-3):
[0040]
[0041] (R, R, R', R')-2 (1.0g, 1.3mmol) and NaBH 4 (1.0g, 26.3mmol) was added into a 150mL three-necked flask, and ethanol (50mL) was added under a nitrogen atmosphere, the temperature was raised to 40°C, and the reaction was stirred at this temperature for 12h to stop the reaction. After cooling to room temperature with nitrogen gas, it was extracted with dichloromethane (150 mL), and washed with distilled water (total 120 mL×3). It was then dried over anhydrous sodium sulfate, filtered, evaporated to remove the solvent under reduced pressure, and dried in vacuo to obtain milky white solid (R, R, R', R')-3 (0.80 g, yield 80%). Melting point: 191-193°C. 1 H NMR (400MHz, CDCl 3 ): δ0.80-0.96(m, 2H), 0.98-1.39(m, 6H), 1....
Embodiment 3
[0047] Compound (R, R, R', R')-3 is used as a chiral ligand for asymmetric catalytic hydrogenation of ketones
[0048]
[0049] (R, R, R', R')-3 (8.0mg, 0.01mmol), RhH(CO)(PPh 3 ) 3 (9.2mg, 0.01mmol) and NH 4 I (22.0mg, 0.15mmol) was added to the reaction tube, 20ml of isopropanol was added in the air, and after stirring at room temperature for 30min, 0.2M KOH / i PrOH solution (115ml, 0.23mmol), stirring was continued for 10min, then acetophenone (0.234ml, 2.0mmol) was added. That is, the mol ratio of each material added is ketone: Rh: ligand: NH 4I:KOH=200:1:1:15:23. The mixed solution was stirred and reacted at 65° C. for 3 h. The reaction solution was analyzed by gas chromatography (chiral chromatographic column: CP-Cyclodextrin-β-2,3,6-M-19, 50m), and the chemical yield and optical purity of the product (S)-phenethyl alcohol were respectively 97% and 86% ee.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com