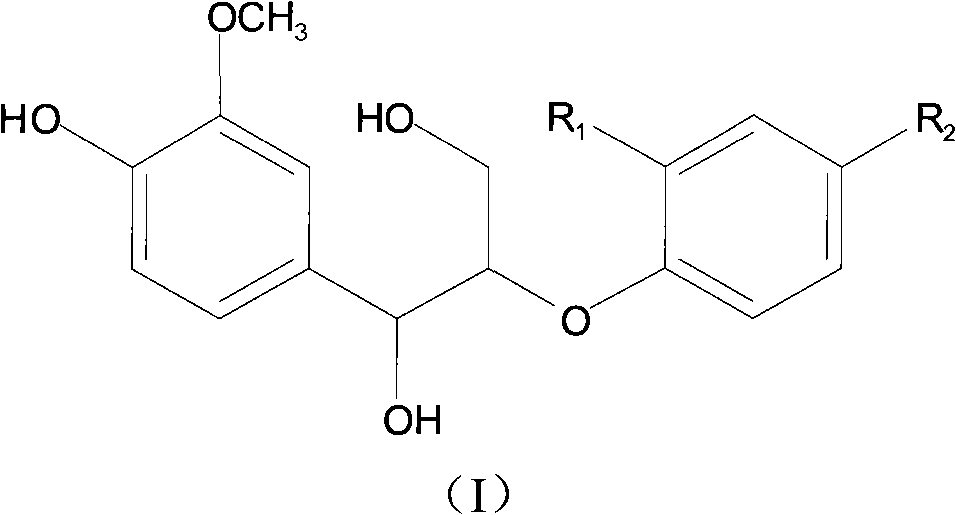
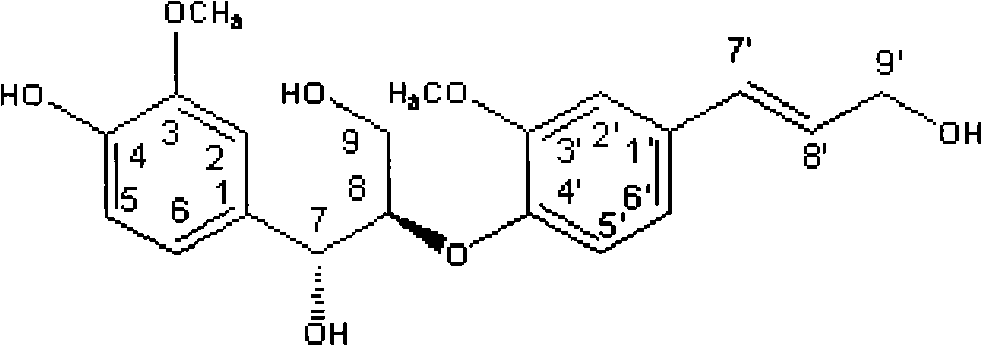
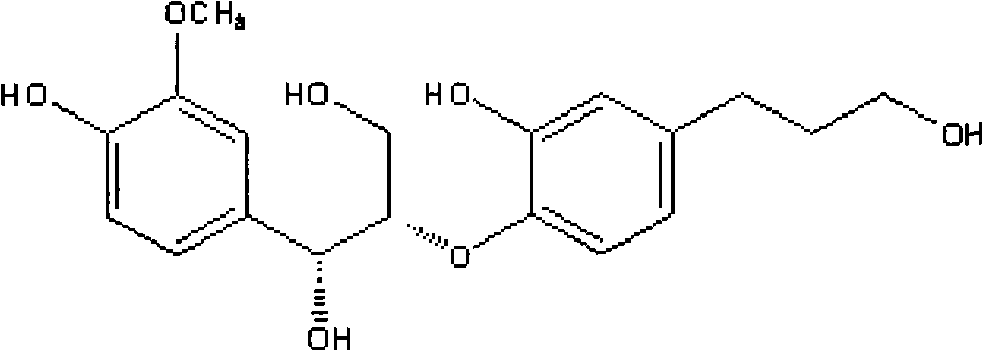
Sambucus williamsii hance lignan compound and preparation method and application thereof
A technology of compounds and lignans, which is applied in the field of elderberry lignan compounds and their preparation, can solve the problems of insufficient research on lignan components and unexplained mechanism of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the preparation method of lignans compound in Elderberry
[0026] Take 50 kilograms of dry stems and branches of elderberry, after proper pulverization, heat and reflux extraction with 10 times the amount of 60% ethanol for 3 times, each time for 2 hours. The extracts were combined, and the solvent was evaporated under reduced pressure to obtain 2160 grams of the total extract of Elderberry. Get 1250 grams of elderberry total extract subsequently, dissolve with suitable amount of water, carry out macroporous resin open column chromatography, use the water of 5 times column bed volumes, 30%, 50%, 95% ethanol-water solution gradient elution successively, The eluents of each part were collected, and the solvent was recovered under reduced pressure respectively to obtain 712.5 g of water eluted fraction, 160 g of 30% ethanol eluted fraction, 125 g of 50% ethanol eluted fraction and 111 g of 95% ethanol eluted fraction. Elderberry 50% ethanol eluting part 20.3...
Embodiment 2
[0027]Example 2: Structural analysis of lignans
[0028] 2.1 Structural Analysis of Compound 1
[0029] Yellow oil (methanol), [α] 27 D -1.5° (c=0.67, MeOH). Combining 1H NMR, 13C NMR and DEPT-135, it was determined that the molecular formula of the compound was C20H24O7, and its degree of unsaturation was calculated to be 9.
[0030] On the 1H NMR (400MHz, in CD3OD) spectrum of compound 1, the downfield region gives 8 sets of hydrogen signals, one of which has a single peak integral value of 2. Although there is signal overlap, according to the chemical shift value and coupling constant, it can still be judged that there are 2 ABX coupling systems and 1 trans double bond coupling, and the ABX coupling systems are δ7.01 (1H, d, J=1.8Hz, H -2), δ6.72 (1H, d, J=8.1Hz, H-5) and δ6.83 (1H, dd, J=8.1, 1.8Hz, H-6); δ6.98 (1H, d, J=1.9Hz, H-2'), δ6.86 (1H, H-5') and δ6.86 (1H, H-6'), the trans double bond is δ6.50 (1H, d, J= 16.0 Hz, H-7') and δ6.22 (1H, dt, J=15.8, 5.8 Hz, H-8...
Embodiment 3
[0042] Example 3: Effects of Lignans on Osteoblast-like UMR 106 Cells
[0043] The in vitro anti-osteoporosis activity of the compound prepared in Example 1 was evaluated by the proliferation and ALP activity of rat osteoblast-like UMR 106 cells.
[0044] 3.1 Experimental samples
[0045] The compound obtained in Example 1 refers to the compound guaiacylglycerol-β-coniferyl ether and D-erythro-1-(4-hydroxyl-3-methoxybenzene) obtained in Example 1. base)-2-[-4-(3-hydroxypropyl)-2-methoxyphenoxy]-1,3-propanediol.
[0046] 3.2 Experimental method
[0047] UMR 106 cells were trypsinized and dispersed, seeded in 96-well plates at a density of 4000 cells / well, and cultured in an incubator at 37°C, humidity 95%, and 5% CO2. After cultured in 10% FBS (fetal bovine serum)-DMEM medium for 2 days, cultured again with 1% Charcoal Stripped FBS (fetal bovine serum removed by activated carbon treatment)-Phenol red-free DMEM (DMEM with phenol red removed) sky. The compound described in E...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com