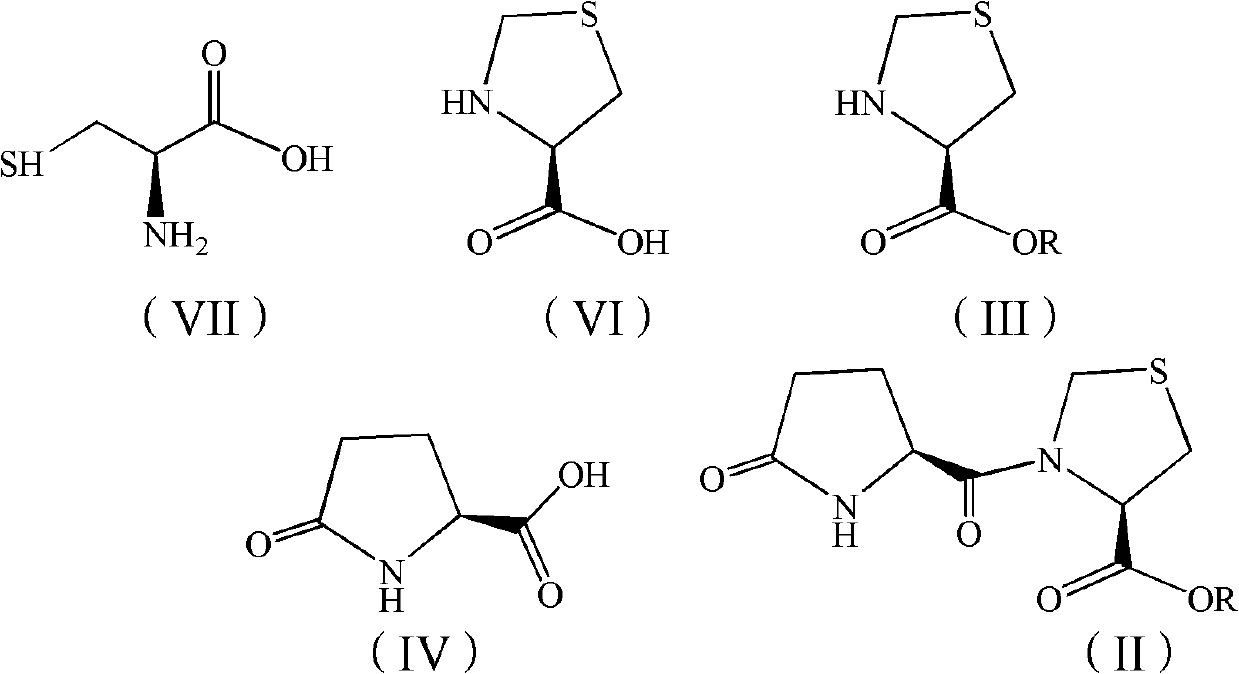
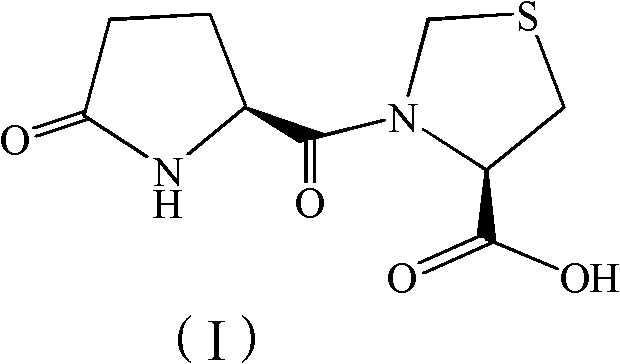
Synthesis method of pidotimod
A synthesis method and condensing agent technology, applied in the direction of peptides, etc., can solve problems such as equipment corrosion, low product yield and purity, and high ester toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: Preparation of L-thiazolidine-4-carboxylic acid methyl ester (III-1)
[0031] Into a 1000mL reaction flask, add 121.2g (1.0003mol) of L-cysteine in turn, add 500mL of purified water and stir to dissolve, then add 86.9g of 38% formaldehyde solution (1.0996mol of formaldehyde), stir for 10h, and cool to 0°C , filtered to obtain colorless needle-like crystals, the filter cake was transferred to a 1000mL three-necked flask, 300mL of purified water was added, the temperature was raised to reflux, and kept stirring for 1h, then the temperature was lowered to 30°C and stirred to precipitate crystals, cooled to 0°C, filtered, and the filter cake After drying, 110.7 g of colorless needle crystals were obtained, that is, L-thiazolidinyl-4-carboxylic acid, and the yield was 83.1%.
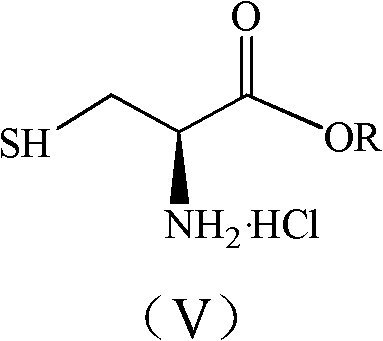
[0032] Add 1200mL of anhydrous methanol to a 3000mL reaction bottle, stir and cool to -2°C, add 240g (3.0573mol) of acetyl chloride dropwise, after the dropwise addition, add 100g (0.75...
Embodiment 2
[0034] Embodiment 2: Preparation of L-thiazolidine-4-carboxylic acid methyl ester (III-1)
[0035] Change 240g of acetyl chloride into 120g (0.4044mol) of bis(trichloromethyl)carbonate, and others are the same as in Example 1, and react to obtain light yellow liquid: 875mL of dichloromethane solution of L-thiazolidine-4-carboxylic acid methyl ester , the mass concentration of L-thiazolidine-4-carboxylate methyl ester is 8.38%.
Embodiment 3
[0036] Embodiment 3: Preparation of L-thiazolidine-4-carboxylic acid methyl ester (III-1)
[0037] Into a 1000mL reaction flask, add 121.2g (1.0003mol) of L-cysteine in turn, add 500mL of purified water and stir to dissolve, then add 33.1g (1.1022mol) of paraformaldehyde, stir for 10h, cool to 0°C, filter Colorless needle-like crystals were obtained, the filter cake was transferred into a 1000mL three-necked flask, 300mL of purified water was added, the temperature was raised to reflux for 1h, the temperature was lowered to stir and crystallize, cooled to 0°C, filtered, and dried to obtain colorless needle-like crystals L-thiazolidinyl- 112.8 g of 4-carboxylic acid, the yield was 84.7%.
[0038] Add 1200 mL of anhydrous methanol to a 3000 mL reaction bottle, stir and cool to -3°C, add 120 g (0.4044 mol) of bis(trichloromethyl)carbonate dropwise, after the addition is complete, add the L-thiazolidinyl-4- Carboxylic acid 100g (0.7509mol), slowly warming up to reflux reaction ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com