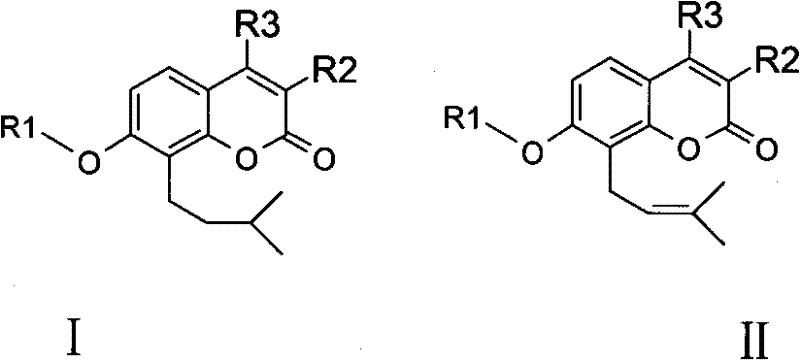
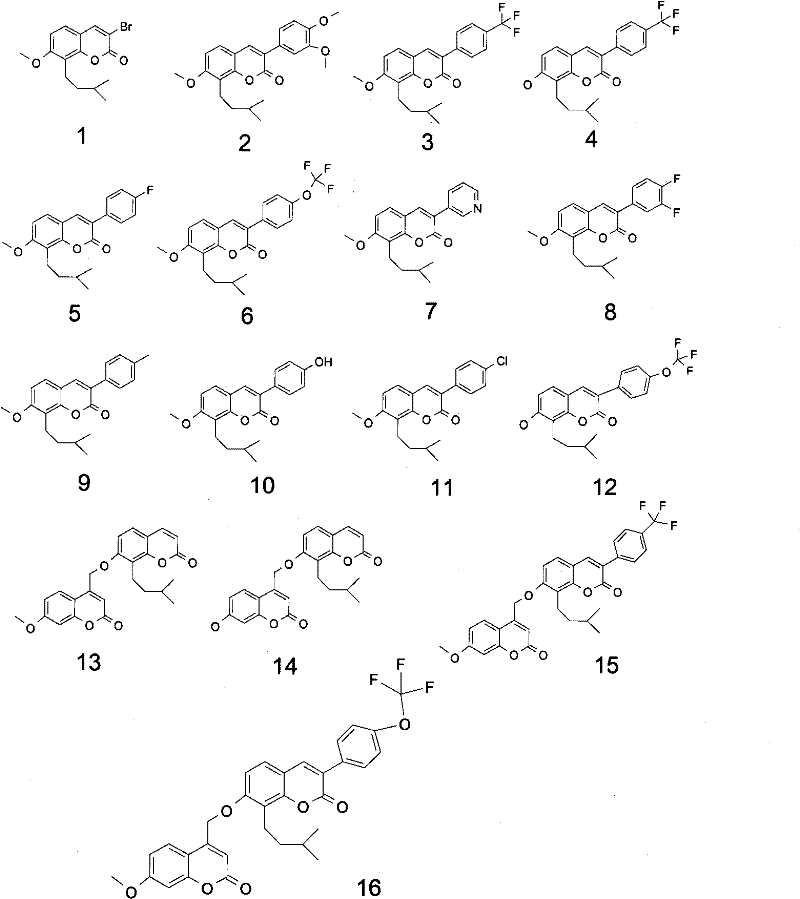
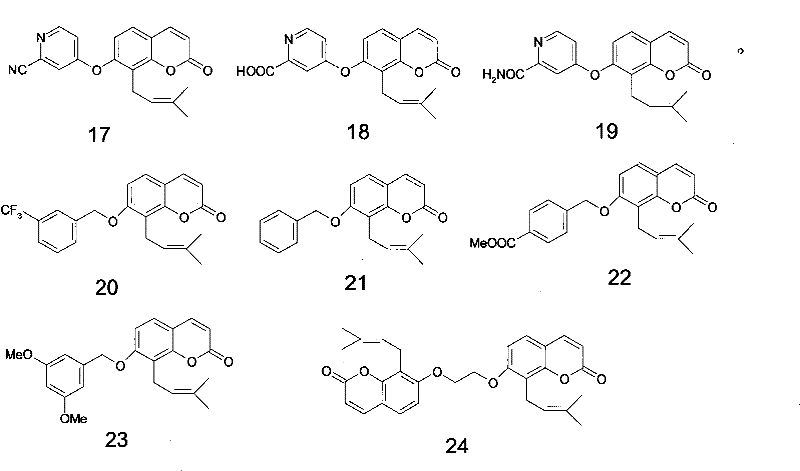
Osthole derivative, its preparation method and its application in preparing medicine for treating breast cancer
A technology of osthole and derivatives, which is applied in the direction of drug combination, antineoplastic drugs, and active ingredients of heterocyclic compounds, etc., which can solve the problems of human body damage, damage to hematopoietic and immune functions, and high price, and achieve fewer synthesis steps and simple reactions , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the preparation of compound 1
[0033] Add 3.0 g (12.3 mmol) of osthole into a round-bottomed flask with an appropriate volume, dissolve it in 20 mL of ethanol solvent, and then add 85 mg (0.35 mmol) of platinum dioxide. The reaction mixture system is degassed under reduced pressure and then filled with hydrogen. (This process was repeated three times), the reaction mixture was stirred and reacted overnight at room temperature in a hydrogen atmosphere, and after the complete disappearance of the reactant was monitored by thin-plate chromatography, about 30 mL of methanol was added to dilute the reaction solution, filtered, the filtrate was concentrated, and the residue was recrystallized to obtain a white solid (hydrogenated snake Bedsin) about 2.8g, yield 91%.
[0034] UPLC-MS (m / z): 1.81min (247.1, M+1)
[0035] 1 H NMR (400MHz, CDCl 3 ): δ7.82 (1H, d, J = 9.0Hz), 7.18 (1H, d, J = 8.4Hz), 6.53 (1H, d, J = 8.4Hz), 6.34 (1H, d, J = 9.4Hz) , 2.81 (2H, t...
Embodiment 2
[0039] Embodiment 2: the preparation of compound 2
[0040] In the flask, add 100 mg (0.31 mmol) of 3-bromohydroosthole and dissolve in 5 mL of dioxane, then add tetrakis (triphenylphosphine) palladium 36 mg (0.031 mmol), cesium carbonate 202 mg (0.62 mmol), Finally, 100 mg (0.31 mmol) of 3,4-dimethoxyphenylboronic acid was added, the reaction system was filled with nitrogen protection, and the reaction was refluxed at 120 ° C for 12 hours. After thin plate chromatography until the reactant completely disappeared, the reaction solution was cooled to room temperature. The organic phase was diluted with ethyl acetate, washed with water and saturated brine successively, dried over anhydrous sodium sulfate and filtered. The organic phase was concentrated under reduced pressure to remove the solvent, and the residue was subjected to silica gel column chromatography (eluent: acetone / petroleum ether=1 / 5) to obtain 50 mg of a white solid (compound 2), with a yield of 42%.
[0041] UP...
Embodiment 3
[0043] Embodiment 3: the preparation of compound 3
[0044] In the flask, add 80 mg (0.25 mmol) of 3-bromohydroosthole and dissolve in 5 mL of dioxane, then add 30 mg (0.025 mmol) of tetrakis(triphenylphosphine) palladium, and 163 mg (0.50 mmol) of cesium carbonate, Finally, 71mg (0.37mmol) of 4-trifluoromethylphenylboronic acid was added, the reaction system was filled with nitrogen protection, and the reaction was refluxed at 120°C for 15 hours. The reaction was monitored by thin-plate chromatography until the reaction was complete. The reaction solution was cooled to room temperature and diluted with ethyl acetate. The organic phase was successively washed with water and saturated brine, dried over anhydrous sodium sulfate and filtered. The organic phase was concentrated under reduced pressure to remove the solvent, and the residue was subjected to silica gel column chromatography (eluent: acetone / petroleum ether=1 / 5) to obtain 40 mg of a white solid (compound 3), with a yi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com