Dengue virus subunit vaccine and preparation method thereof
A subunit vaccine, dengue virus technology, applied in the field of dengue virus subunit vaccine and its preparation, can solve the problems of large differences in mouse protection ability, large difference in protection ability of DV type, and unfavorable promotion, etc. The effect of low mammalian cell expression system, convenient purification route and cost control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
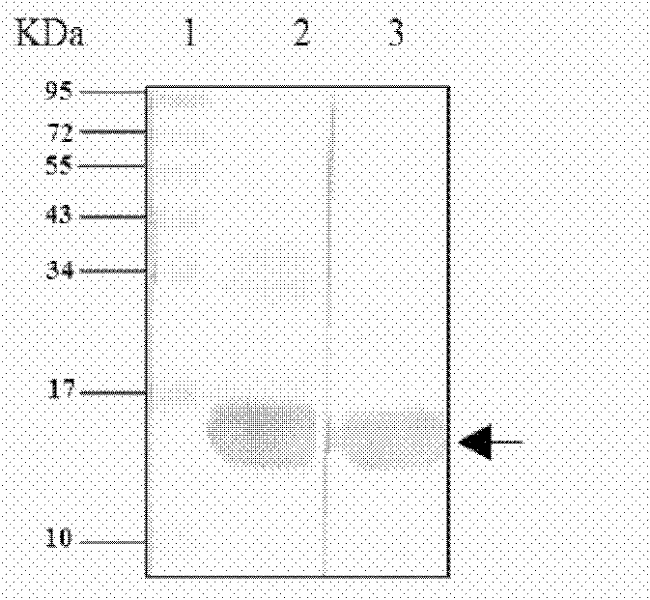
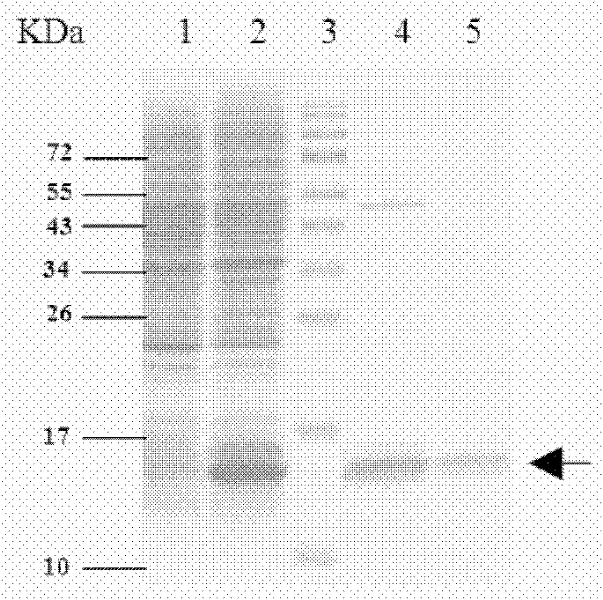
[0076] After obtaining the recombinant expression plasmid ligation product, a conventional method is to introduce it into the target bacterium, and a preferred method is to use antibiotics (such as ampicillin) corresponding to the resistance gene carried by the plasmid to screen the bacteria transformed by the plasmid, Obtain bacterial clones with stable resistance, amplify the desired clones, induce them with the inducer IPTG, analyze the expression bands by SDS-PAGE electrophoresis, and determine the bacterial clones expressing the target protein.
Embodiment 3
[0078] The clones screened in 2 were cultured, added with 0.5 mM inducer IPTG, induced at 30° C. for 4 hours, collected by centrifugation, and ultrasonically disrupted to prepare recombinant protein inclusion bodies.
[0079] 4. the purification of dengue virus subunit vaccine protein (see embodiment 4)
[0080] The inclusion body was dissolved in 8M urea, and the supernatant was collected by centrifugation, purified by Ni-NTA affinity chromatography column and cation exchange strain respectively, and the elution peak was collected, dialyzed into the preservation solution for later use.
[0081] Conventional reagents used in the present invention are commercially available analytical reagents.
Embodiment 1
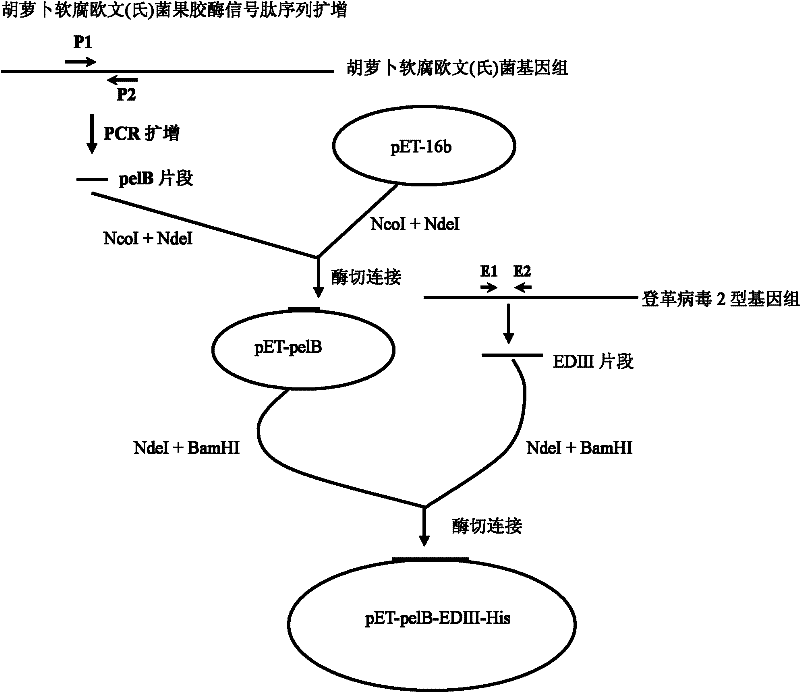
[0083] Construction process of dengue virus subunit vaccine recombinant expression plasmid
[0084] 1. PCR amplification of the leader peptide (pelB fragment) sequence
[0085] (1) The guide peptide is the Erwinia carotovora pectinase signal peptide. Design a pair of oligonucleotide fragments according to the signal peptide sequence shown in SEQ NO 1 (Lei SP, et al, J Bacteriol.1987; 169 (9): 4379-4383): P1: GCAAATACCTGCTGCCGACCGCT (NcoI site in bold) (SEQ ID No: 3), P2: GGCCATCGCGGTGGGC (NdeI site in bold) (SEQ ID No: 4) was synthesized by the DNA Synthesis Department of Shenzhen Huada Gene Company.
[0086] (2) Preparation of Genomic DNA of Erwinia carotovora Genome extraction kit is a Promega product, and the operation is carried out according to the company's instructions.
[0087] (3) PCR amplification Use the DNA extracted in (2) as a template, and carry out PCR amplification with P1-P2 primers, and the reaction is as follows:
[0088]
[0089]After mixing, plac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com