Blue luminescent material for white light LED and novel preparation method thereof
A blue fluorescence, white light technology, applied in the field of phosphate blue fluorescent materials, can solve the problems of long preparation time, high reaction temperature, difficult to control conditions, etc., and achieve stable physical and chemical properties, good chemical stability, and conditions Easy-to-control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
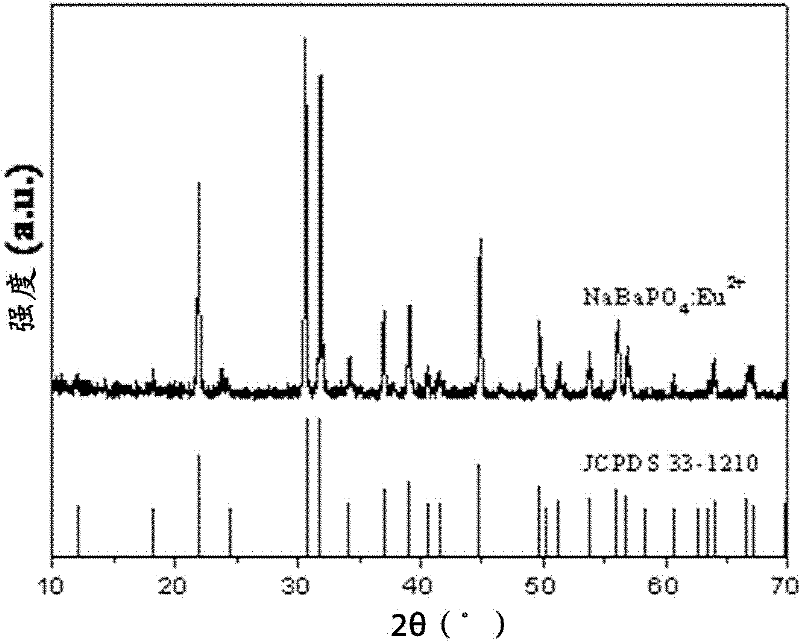
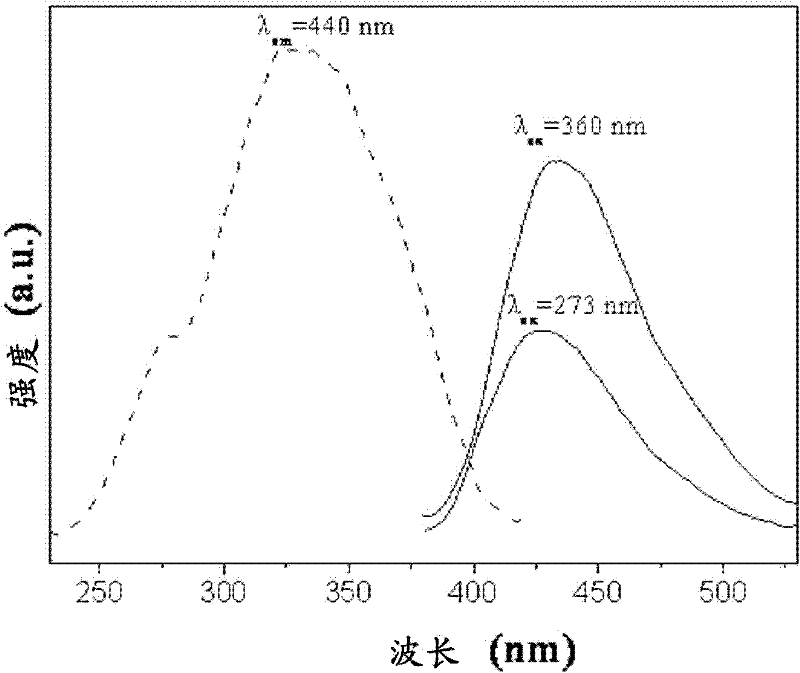
Image
Examples
Embodiment 1
[0042] Example 1: NaBa 0.999 PO 4 :0.001Eu 2+ preparation of
[0043] Weigh each raw material with stoichiometric ratio according to the above chemical composition formula, that is, weigh 0.0026g of Eu 2 o 3 , dissolved in concentrated nitric acid to obtain a rare earth metal europium ion solution; weigh 1.2749g of NaNO 3 , 3.9162g of Ba(NO 3 ) 2 , 1.7255g of NH 4 h 2 PO 4 And the boric acid of 0.0464g is dissolved with deionized water to obtain the corresponding mixed ion solution.
[0044] The rare earth ion solution and the mixed ion solution were mixed and electromagnetically stirred for 5 minutes. Then 3.2432 g of urea was added, and electromagnetic stirring was continued for 5 minutes. Then a mixed solution containing rare earth metal europium ions is obtained. The solution containing the rare earth metal europium ions was poured into a porcelain crucible, and the moisture in the solution was dried in an oven at 80°C. Then the porcelain crucible was placed i...
Embodiment 2
[0048] Example 2: NaBa 0.995 PO 4 :0.005Eu 2+ preparation of
[0049] Weigh each raw material with stoichiometric ratio according to the above chemical composition formula, that is, weigh 0.0132g of Eu 2 o 3 , dissolved in concentrated nitric acid to obtain a rare earth metal europium ion solution; weigh 1.2749g of NaNO 3 , 3.900g of Ba(NO 3 ) 2 , 1.7255g of NH 4 h 2 PO 4 And the boric acid of 0.0464g is dissolved in deionized water to obtain a mixed solution of corresponding ions.
[0050] The rare earth metal europium ion solution and the mixed ion solution were mixed and electromagnetically stirred for 5 minutes. Then 3.2432 g of urea was added, and electromagnetic stirring was continued for 5 minutes. Then a mixed solution containing rare earth metal europium ions is obtained. The mixed solution containing rare earth metal europium ions was poured into a porcelain crucible, and the moisture in the solution was dried in an oven at 80°C. Then place it in a muffl...
Embodiment 3
[0052] Example 3: NaBa 0.99 PO 4 :0.01Eu 2+ preparation of
[0053]Weigh each raw material with stoichiometric ratio according to the above chemical composition formula, that is, weigh 0.0264g of Eu 2 o 3 , dissolved in concentrated nitric acid to obtain a rare earth metal europium ion solution; weigh 1.2749g of NaNO 3 , 3.8809g of Ba(NO 3 ) 2 , 1.7255g of NH 4 h 2 PO 4 And the boric acid of 0.0464g is dissolved in deionized water to obtain the corresponding ion mixed solution.
[0054] The rare earth metal ion solution and the corresponding ion mixed solution were mixed and electromagnetically stirred for 5 minutes. Then 3.2432 g of urea was added, and electromagnetic stirring was continued for 5 minutes to obtain a mixed solution containing rare earth metal europium ions. The mixed solution containing rare earth metal europium ions was poured into a porcelain crucible, and the moisture in the solution was dried in an oven at 80°C. Then place it in a muffle furnac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com