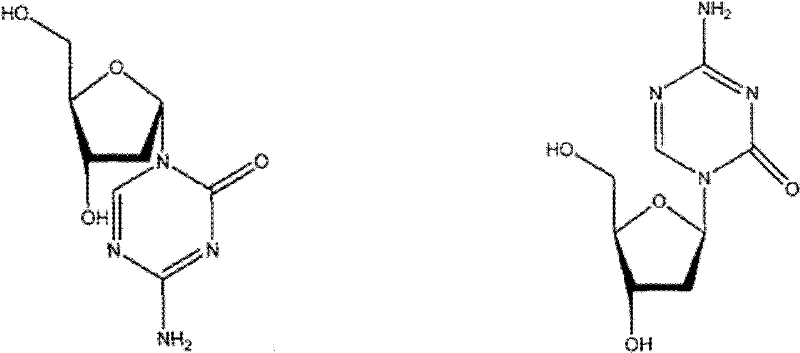
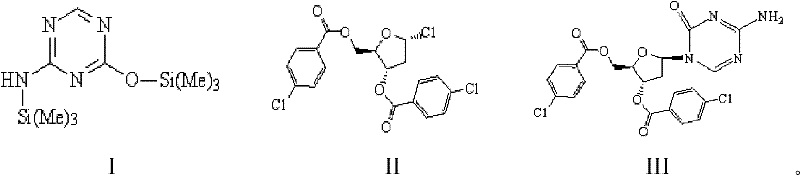
Synthetic method of decitabine
A synthetic method, the technology of decitabine, applied in the field of drug synthesis, can solve the problem of large α-configuration isomer of chlororibose-azacytosine, insufficient purity of β-type chloro-ribose-azacytosine, Solve the problems of high impurity content in the α-alpha configuration, and achieve the effects of reducing the difficulty of purification, low price, and beneficial to industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Detection of the purity of β-chlororibose azacytosine
[0038] Chromatographic conditions: Octadecylsilane-bonded silica gel is used as filler, methanol-water=78:22 is used as mobile phase, flow rate is 1.0 mL / min, and detection wavelength is 240 nm.
[0039] Preparation of the reference substance solution: Weigh an appropriate amount of the reference substance β-chlororibose azacytosine (purchased from Jiangsu Handerson Pharmaceutical Technology Co., Ltd.), dissolve it with methanol and dilute to the mark to make a solution containing 1.0 mg per 1 mL. solution.
[0040] Preparation of the test solution: Weigh an appropriate amount of the test sample, dissolve it with methanol and dilute to the mark to make a solution containing 1.0 mg per 1 mL.
[0041] Determination method: Take 10 μL of the reference substance solution and the test solution respectively and inject them into the liquid chromatograph, record the chromatograms for 30 minutes, and calculate t...
Embodiment 2
[0042] Embodiment 2: Detection of the purity of β-type decitabine
[0043]Chromatographic conditions: Octadecylsilane bonded silica gel as filler, water (A)-methanol (B) as mobile phase, linear gradient elution, elution sequence as shown in Table 1, 1.0mL / min, detection The wavelength is 240nm, and the column temperature is 30°C.
[0044] Table 1 water (A)-methanol (B) elution sequence table
[0045] time (min)
Mobile phase A(%)
Mobile phase B(%)
0
100
0
4
100
0
15
97
3
35
40
60
55
40
60
55.1
100
0
60
100
0
[0046] Preparation of reference solution: Accurately weigh about 20.0 mg of β-type decitabine (purchased from Nanjing Zhongyuan Science and Technology Co., Ltd.), place it in a 10 mL measuring bottle, dissolve it with dimethyl sulfoxide, and dilute to the mark to prepare Each 1mL contains 1.0mg of the solution.
[0047]...
Embodiment 3
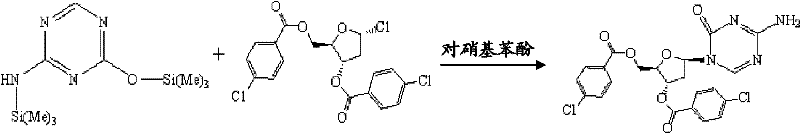
[0050] Under nitrogen protection, after dissolving 60.0g (0.23mol) 2,4-bis-(trimethylsilyl)-5-azacytosine with 500mL dichloromethane, put it into a 1L three-necked flask and stir, add 85g (0.20 mol) 1-chloro-3,5-di-p-chlorobenzoyloxy-2-deoxy-D-ribose and 10.5g (0.075mol) p-nitrophenol, then rinse the addition funnel with 100mL dichloromethane, 30°C Stir the reaction for 12 hours, concentrate the reaction solution to dryness at 30°C under reduced pressure, dissolve the oily substance with 1000mL dichloromethane, then pour it into 1200mL pre-cooled saturated sodium carbonate solution under stirring, separate the layers, and wash the water layer with 500mL dichloromethane Methane was extracted twice, the organic layers were combined, the organic layer was washed with water, dried overnight with anhydrous sodium sulfate, filtered with diatomaceous earth, and concentrated to dryness under reduced pressure to obtain 97 g of chlororiboazacytosine shown in formula III, with a yield of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com