Preparation method of organic-inorganic hybrid monolithic capillary column
A capillary monolithic column and organic technology, applied in chemical instruments and methods, separation methods, solid adsorbent liquid separation, etc., can solve the problems of few types of organosilanes, long reaction time, cumbersome operation, etc., and achieve uniform pore size distribution and preparation Simple process and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
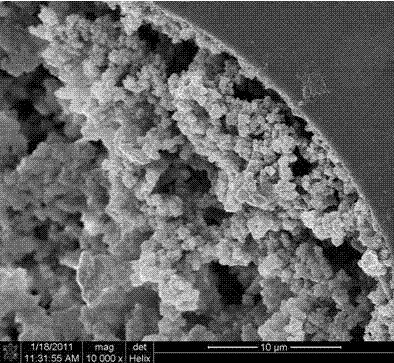
Image
Examples
Embodiment 1
[0025] 1. Column pretreatment
[0026] First rinse the capillary empty column with 0.1mol / L HCl solution for about 1h, then rinse with deionized water for 30min, then rinse with 0.1mol / L NaOH for 4h, then rinse with deionized water for 30min, and finally with methanol for 30min, nitrogen Blow dry and set aside.
[0027] 2. Silanization
[0028] Inject a mixture of anhydrous methanol and methacryloxypropyltrimethoxysilane (γ-MAPS) with a volume ratio of 1:1 into the pretreated capillary, react at 60°C for 24 hours, and then use methanol for 30 minutes. Dry with nitrogen at 60°C.
[0029] 3. In-column synthesis
[0030] Accurately weigh 38 mg of polyethylene glycol 10000 (PEG 10000) into a round-bottom flask, add 0.35 mL of 0.01 mol / L acetic acid, 132 μL of tetramethoxysilane (TMOS), and 22 μL of γ-MAPS on ice. Stir vigorously for 1 h in a water bath, and the mixture became transparent and viscous. Add 20 mg of 4-vinylbenzeneboronic acid (VPBA), 80 μL of diethylene glycol and 1 mg of ...
Embodiment 2
[0034] Accurately weigh 38 mg of PEG 10000 into a round bottom flask, add 0.35 mL of 0.01 mol / L acetic acid, 132 μL of TMOS, and 44 μL of vinyl trimethoxysilane (VTMS) respectively, and stir vigorously for 1 h under ice-water bath conditions. The mixture is transparent and viscous. Add 12.5 mg of VPBA, 10 μL of acetone, and 1 mg of AIBN to the above mixture, and vortex repeatedly for 20 minutes (to prevent the ultrasonic water from overheating during the ultrasonic process) until the mixture is completely dissolved. The viscous mixture was injected into a 75 μm inner diameter capillary tube after silanization to a position of 25 cm, and the two ends were sealed in a 45 ℃ water bath to react for 12 h, and then transferred to a 75 ℃ water bath for another 12 h. After the reaction is over, take it out and cool it to room temperature. Use a high-pressure constant-flow pump to flush with 100% methanol at a low flow rate for 40 minutes to remove unreacted functional monomers and some...
Embodiment 3
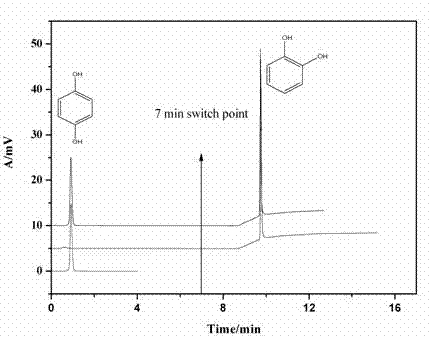
[0036] In micro-column liquid chromatography (μHPLC) mode, with pH 8.0, ion concentration 20 mmol / L, acetonitrile:phosphate (30 / 70, v / v) buffer as mobile phase A, running time 0-6 min ; With pH 3.6, ion concentration 20 mmol / L, acetonitrile: acetate (30 / 70, v / v) buffer as mobile phase B, running time starts from 7 min; pump flow rate is 0.05 mL / min; detection wavelength It is 214 nm; under the conditions of mobile phase A, resorcinol (0.1 mg / mL) is directly eluted without being retained on the monolithic column, while the 1,2-cis catechol (0.1 mg / mL) mg / mL) is adsorbed on the column due to the formation of a reversible covalent bond with the boronic acid group. When the mobile phase is switched to B condition, catechol is eluted. The mixture of the two can be separated efficiently and selectively on the organic-inorganic hybrid capillary monolithic column prepared in Example 1. The chromatographic separation diagram is as follows image 3 Shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com