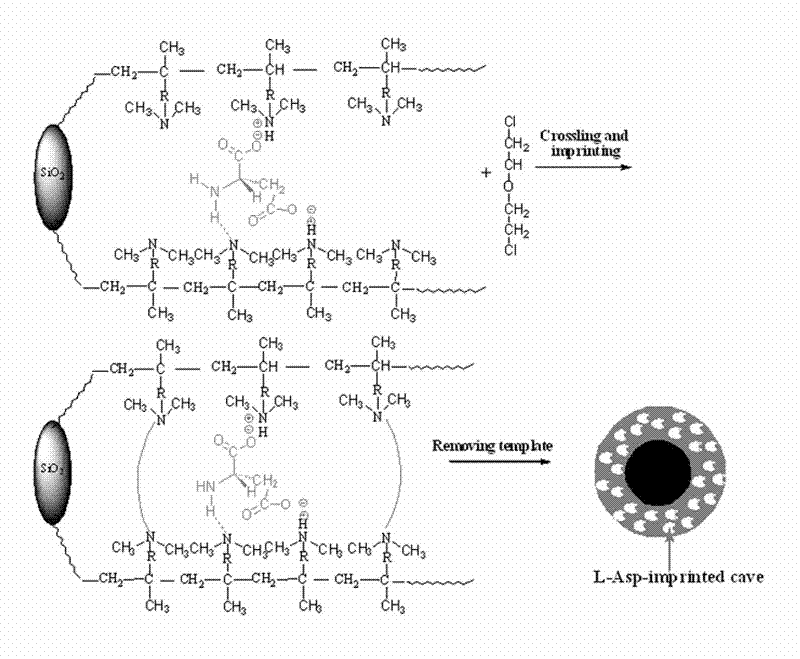
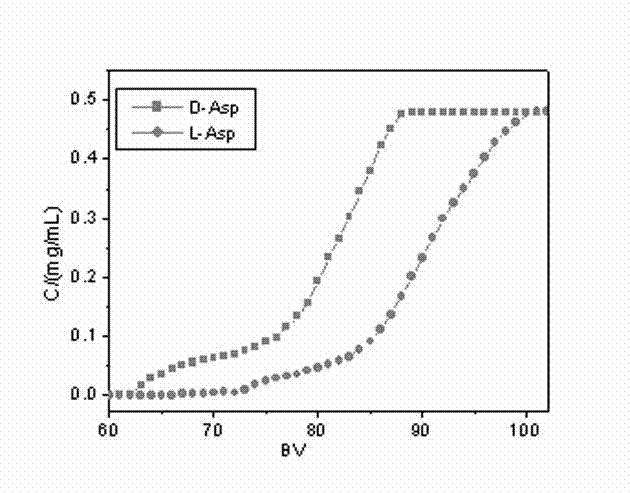
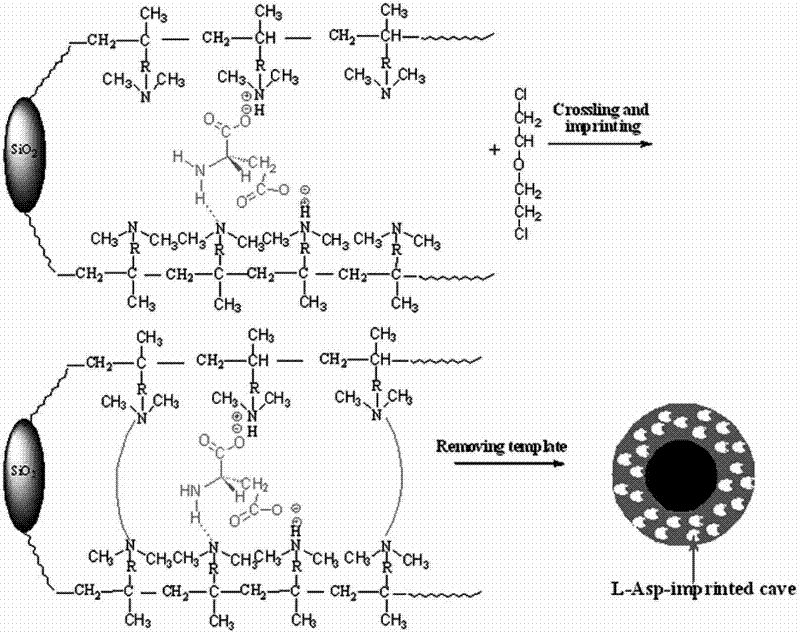
Preparation method of new material for separating chiral aspartic acid
A technology for aspartic acid and new materials, which is applied in the field of preparation of new materials for splitting chiral aspartic acid, can solve the problems of difficulty in achieving industrial production scale and low efficiency of splitting chiral amino acids, and improve mechanical The effect of intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] A preparation method of a new material for splitting chiral aspartic acid, comprising the following steps:
[0043] (1) Chemical surface modification of silica gel: add 10g of activated silica gel to 130ml of water solvent, and add 13ml of coupling agent γ-(methacryloyloxy)propyltrimethoxysilane, react at 50°C for 22h , the product after suction filtration was repeatedly washed with ethanol and dried in vacuum to obtain surface-modified silica gel particles MPS-SiO 2 ;
[0044] (2), chemically grafted polymethacrylic acid on the surface of silica gel particles: add 1.3g of modified silica gel particles MPS-SiO to a four-necked flask 2 , then add 80mL of water and 4.5mL of monomer DMAEMA, pass nitrogen for 30min to remove the air in the system, then raise the temperature of the system to 40°C, add the initiator ammonium persulfate, the amount of ammonium persulfate is the monomer mass 1% of the graft polymerization reaction at 75 ° C for 10 h, suction filtration, to ob...
Embodiment 2
[0047] A preparation method of a new material for splitting chiral aspartic acid, comprising the following steps:
[0048] (1) Surface chemical modification of silica gel: Add 13g of activated silica gel to 150ml of water solvent, and add 10ml of coupling agent trimethoxyallylsilane, react at 50°C for 24h, and the product after suction filtration is washed with ethanol After repeated washing and vacuum drying, the surface-modified silica particles MPS-SiO 2 ;
[0049] (2), chemically grafted polymethacrylic acid on the surface of silica gel particles: add 1.5g of modified silica gel particles MPS-SiO to a four-necked flask 2 , then add 90mL of water and 5mL of monomer DMAEMA, pass nitrogen for 30min to remove the air in the system, then raise the temperature of the system to 40°C, add the initiator ammonium persulfate, the amount of ammonium persulfate added is 1% of the monomer mass 1.1%, carry out graft polymerization at 75°C for 11 hours, and filter with suction to obtain...
Embodiment 3
[0052] A preparation method of a new material for splitting chiral aspartic acid, comprising the following steps:
[0053] (1) Surface chemical modification of silica gel: Add 15g of activated silica gel to 100ml of water solvent, and add 15ml of coupling agent trimethoxyvinylsilane, react at 50°C for 20h, and the product after suction filtration is repeated with ethanol Washed and dried in vacuum to obtain surface-modified silica particles MPS-SiO 2 ;
[0054] (2), chemically grafted polymethacrylic acid on the surface of silica gel particles: add 1g of modified silica gel particles MPS-SiO to a four-necked flask 2 , then add 100mL of water and 4mL of monomer DMAEMA, pass nitrogen for 30min to get rid of the air in the system, then raise the temperature of the system to 40°C, add the initiator ammonium persulfate, the amount of ammonium persulfate added is the weight of the monomer 1.2%, carry out graft polymerization reaction at 75°C for 12h, and filter with suction to obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com