Spirofluorene xanthene phosphine oxide electro-phosphorescent main materials and synthesis and application methods thereof
A technology of spirofluorene xanthene phosphine and xanthene phosphine is applied in the field of synthesis of diphenyl phosphine series compounds, which can solve the problem of low triplet energy level, poor thermal stability and morphological stability, and limited application. and other problems, to achieve the effects of low cost of raw materials, high thermal stability, and simple and easy synthesis method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0070] Utilize the preparation method of one-pot spirocycle (such as literature: Org.Lett.2006 (8), 2787-2790), under the protection of inert gas, 10 times of equivalents of phenol (or p-bromophenol) and 1 times of equivalents of 2 , 7-dibromo-9-fluorenone (or 9-fluorenone) at 150 ° C, and then add 4 times the equivalent of methanesulfonic acid to catalyze the reaction for 12 hours. The monomer product was obtained by silica gel column chromatography.
[0071] (2) Synthesis of organophosphorus compounds of spirofluorene xanthene
[0072] Under the protection of an inert gas, dissolve 1 times the equivalent of the reactant (bromine-containing spirofluorene xanthene) in tetrahydrofuran solution (THF), and slowly add 1.5 times the equivalent of n-butyl at low temperature (-78°C) Lithium, using n-butyllithium and the reactant (bromine-containing spirofluorene xanthene) for lithium halide exchange, so that the spirofluorene xanthene forms a lithium salt, and then reacts at low tem...
Embodiment 1
[0082] (1) Synthesis of 2-bromo-spirofluorene xanthene
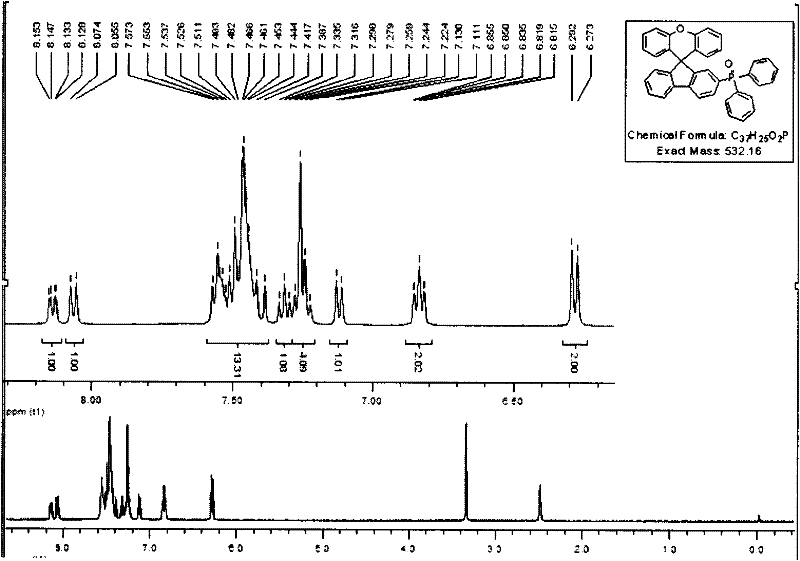
[0083] Add dry 2-bromofluorenone (5.0g, 19.3mmol), phenol (18.2g, 192.8mmol) and methanesulfonic acid (5mL, 77.2mmol) into a two-necked round-bottomed flask equipped with a magnet, and add Spherical condenser, closed system, protected from light, replaced nitrogen 3 times, placed in an oil bath, heated to 150°C, and reacted for 5 hours. At the end of the reaction, water (200 mL) was added and stirred. Sodium hydroxide (7.7 g, 192.8 mmol) was added to adjust the pH value to basic, and the solid crude product was obtained by suction filtration. The crude product was eluted with petroleum ether as eluent by silica gel column chromatography to obtain a white solid product (4.8 g). The yield was 60%. 1 H NMR (400MHz, CDCl 3 , ppm) δ: 7.783-7.763 (d, 8.0Hz, 1H), 7.667-7.646 (d, 8.4Hz, 1H), 7.5-7.475 (d, 8.0Hz, 1H), 7.406-7.366 (t, 7.6Hz, 1H), 7.285-7.265(d, 8.0Hz, 1H), 7.246-7.19(m, 5H), 7.169-7.15(d, 7.6Hz, 1H), 6.819-6....
example 2
[0100] Example two: the ultraviolet absorption spectrum of (the product in embodiment 1), photoluminescence spectrum, spectral thermal stability and quantum efficiency measurement
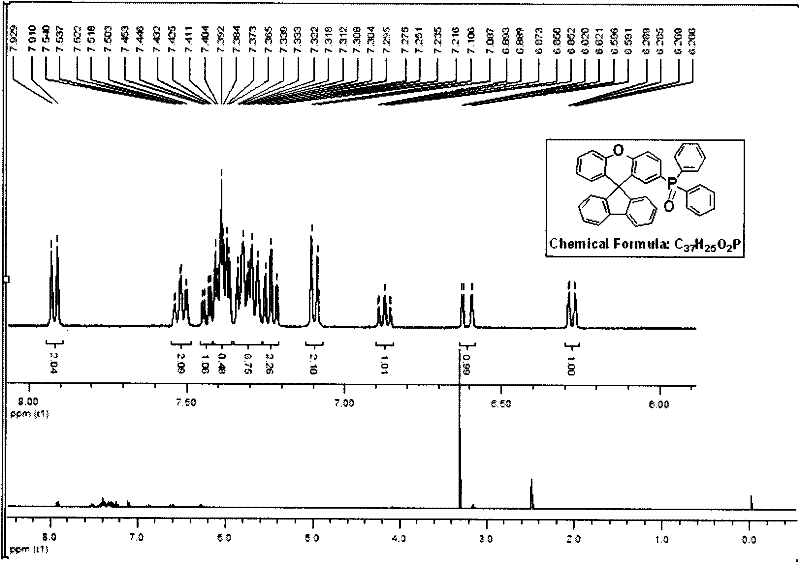
[0101] (1) Dissolve SFX2PO in dichloromethane dilute solution, and use Shimadzu UV-3150 ultraviolet-visible spectrometer and RF-530XPC fluorescence spectrometer to measure the absorption and emission spectra. The photoluminescence spectrum was measured at the maximum absorption wavelength (312 nm) of ultraviolet absorption. The solid film is formed by dropping the solution on a transparent glass plate after the solvent evaporates. The maximum absorption peak of the SFX2PO solution is 312nm, and the emission peaks of the fluorescence spectrum are 319nm and 332nm. The maximum emission wavelength of the solid film is 369nm. See attached for details Figure 5 .
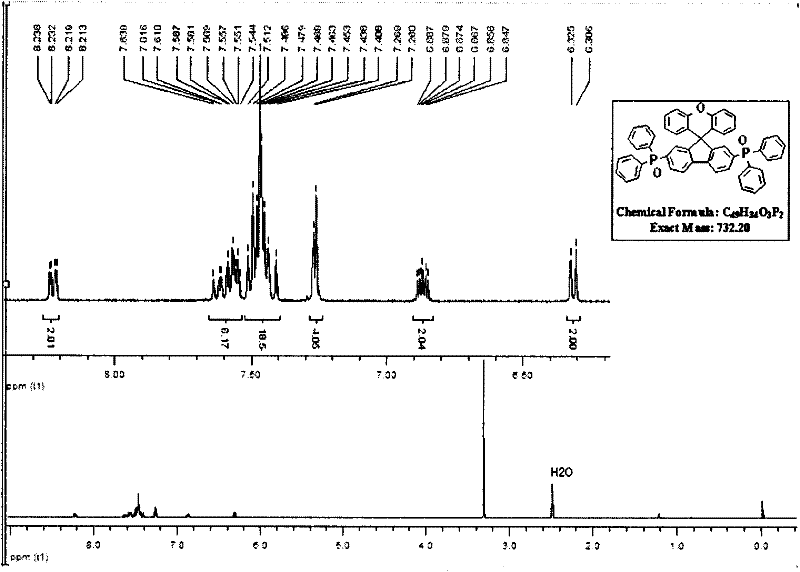
[0102] (2) Dissolve SFX27PO in dichloromethane dilute solution, and use Shimadzu UV-3150 ultraviolet-visible spectrometer and RF-530XPC f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com