High-efficient expression and application of amidohydrolase
A technology of amidohydrolase and hydrolase, which is applied in the fields of molecular biology and enzyme engineering, can solve problems such as the research of gene expression systems, achieve the effects of reducing energy consumption, overcoming harsh conditions, and realizing green environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
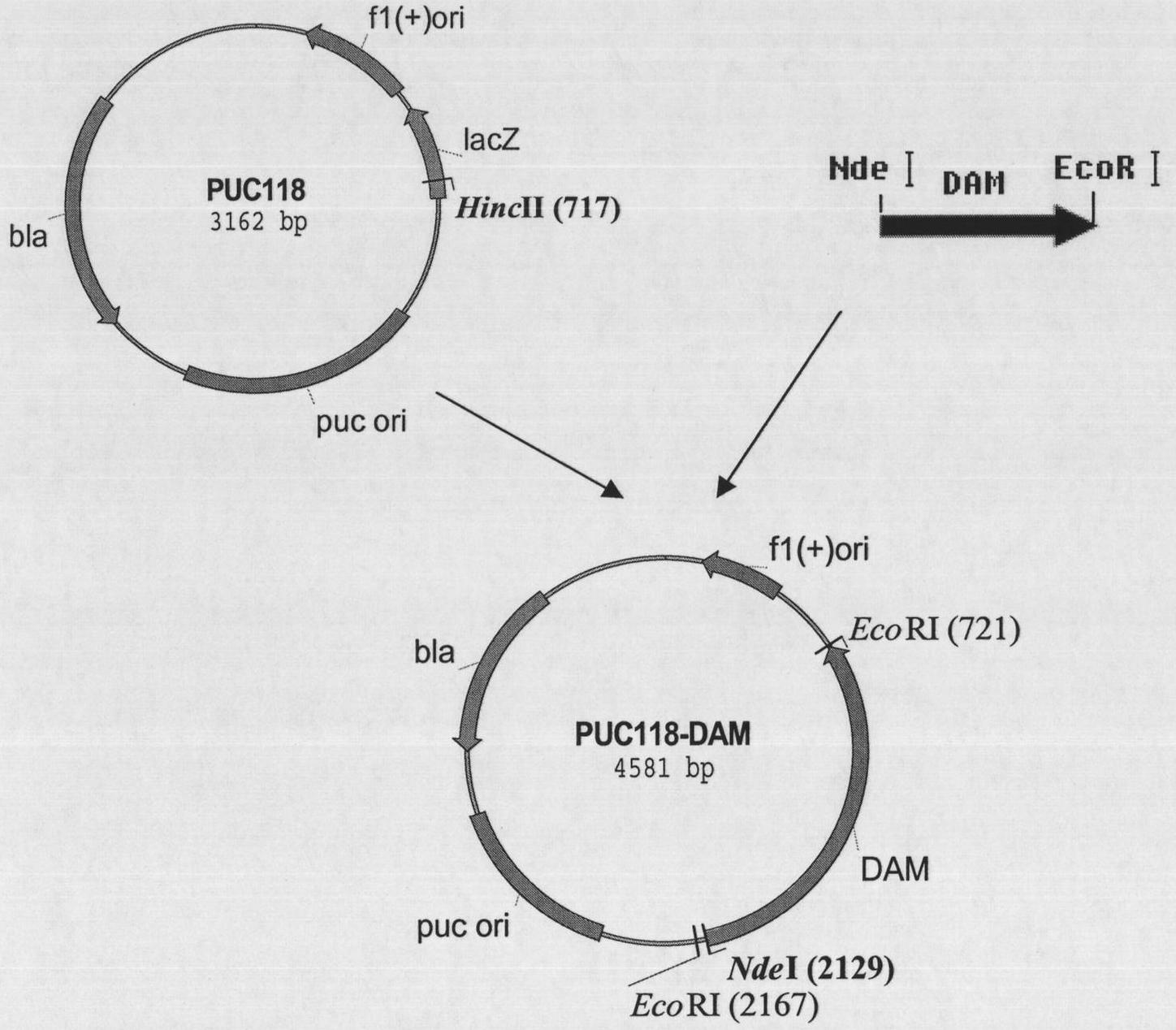
[0049] Embodiment 1: clone stereoselective amidohydrolase gene (DAM gene) from Delphi bacterium
[0050] 1. Genomic DNA acquisition
[0051] Genomic DNA of Delftia tsuruhatensis (CCTCCNo.M205114) was extracted using a genome extraction kit.
[0052] 2. Primer design and amplification of target gene DAM
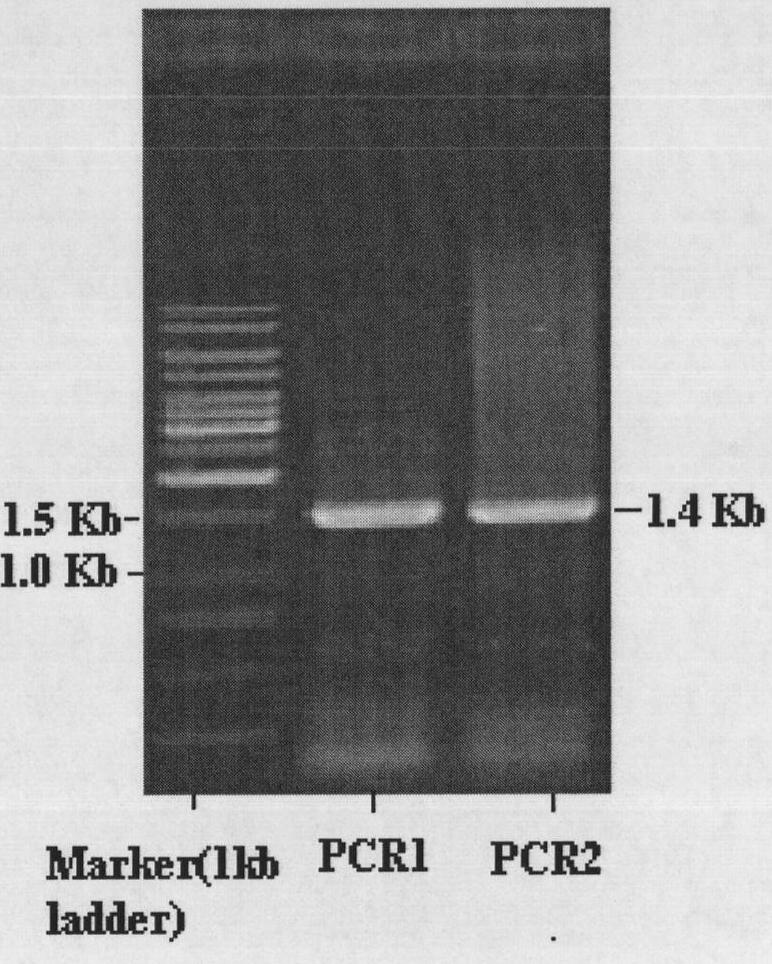
[0053] According to the amidohydrolase gene from Delftia acidovorans reported in GenBank (gi|160361034:5879135-5880535, researchers speculate that the gene has the function of amidohydrolase), two PCR primers were designed:
[0054] Primer A (5'to 3'): TCT CAT ATG ATG AAC GAC AGC GAA CTGCAC CAT CTC GAA CT
[0055] Primer B (5'to 3'): CTA GAA TTC TCA GGC AGC AGG GTG CTGTCT GTG CCA GT
[0056] A primer (primer A) at the 5' end of the designed gene introduced an Nde I restriction site; a primer at the 3' end of the gene (primer B) introduced an EcoR I restriction site.
[0057] Using genomic DNA as a template, add a certain amount of primers A and B, Taq DNA polymerase ...
Embodiment 2
[0069] Embodiment 2: Construction of expression vector PET24a (+)-DAM and genetically engineered bacteria Escherichia coli (Escherichia coli) BL21 (DE3) / PET24a (+) DAM
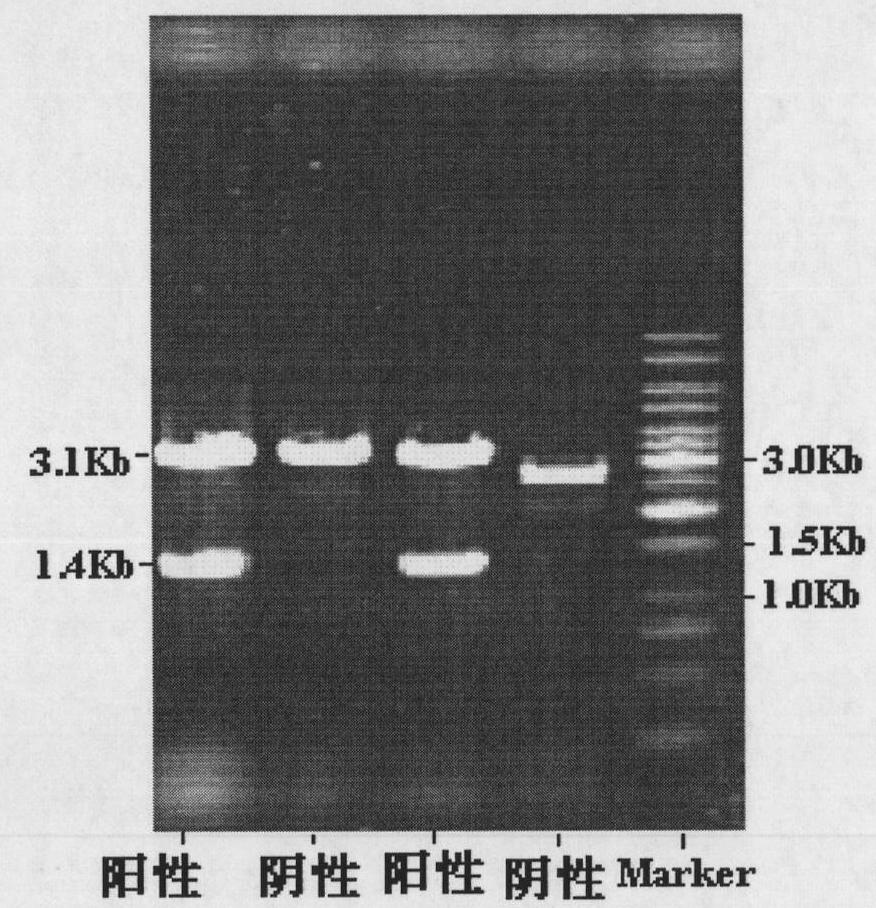
[0070]After the pUC118-DAM plasmid and PET24a(+) were digested by EcoR I and Nde I respectively, the digested products were separated by 0.8% agarose gel electrophoresis, and the DNA fragments of about 1400bp and 5260bp were recovered from the gel, and T4DNA Ligase ligation, the resulting plasmid is called PET24a(+)-DAM plasmid, the ligation product is transformed into competent Escherichia coli (Escherichiacoli) BL21(DE3), and positive clones are obtained by screening on a kanamycin-resistant plate and subjected to EcoR I And Nde I double enzyme digestion identification, proved to contain the correct insert (such as Figure 4 shown), thereby obtaining the genetic engineering strain Escherichia coli (Escherichiacoli) BL21(DE3) / PET24a(+) DAM containing amidohydrolase of the present invention. The construction ...
Embodiment 3
[0071] Example 3: Fermentative expression of exogenous stereoselective amidohydrolase engineered bacteria BL21(DE3) / PET24a(+) DAM
[0072] The seed medium is LB medium, and the inoculation amount is 0.5-6%. The fermentation medium is TB medium: 24g / L yeast extract, 12g / L peptone, 4g / L industrial glycerol, 0.1M phosphate buffer, pH=7.5. When the seed OD600=1 or so, add 150ml seed fermentation liquid to 3000ml fermentation medium, start culturing at 37°C, when OD600=2, add 1% α-lactose for induction, induction temperature is 28°C, after 5 hours , add 1% α-lactose for the second time, when the dissolved oxygen rises to a higher value and no longer drops, put the tank.
[0073] The growth curve of the fermentation process is shown in Image 6 , Compared with the fermentation process of the host strain Delftia tsuruhatensis, the fermentation time is shortened and the biomass is increased. Under the above culture conditions, the enzyme activity can reach about 3000U / L.min when pu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com