Inclusion complex of cyclodextrin/fe3o4 magnetic nanocomposite with ibuprofen
A magnetic nanometer and cyclodextrin technology, applied in the direction of drug combination, organic active ingredients, non-central analgesics, etc., can solve the problem of frequent medication to maintain therapeutic concentration, strong gastrointestinal stimulation, dissolution rate and bioavailability Low-level problems, to achieve significant solubilization effect and high solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: β-Cyclodextrin / Fe 3 o 4 Preparation of magnetic nanocomposites
[0027] (1) Fe 3 o 4 Preparation of Magnetic Nanoparticles (MNPs)
[0028] Weigh FeCl with a molar ratio of 1:1 2 .4H2 O and FeCl 3 .6H 2 O (total iron concentration 0.3mol / L) is placed in a 250mL round bottom flask, add an appropriate amount of double distilled water 194mL, fully stir and dissolve at a speed of 600r / min at a temperature of 30°C, and add concentrated ammonia water (25-28%) dropwise at the same time When the pH is 9 to complete the precipitation, heat up to 80°C and mature for 30 minutes. The reactant is naturally cooled to room temperature, washed repeatedly with double distilled water and ethanol, separated by a permanent magnet, and vacuum-dried at 70°C for 24 hours to obtain Fe 3 o 4 magnetic nanoparticles.
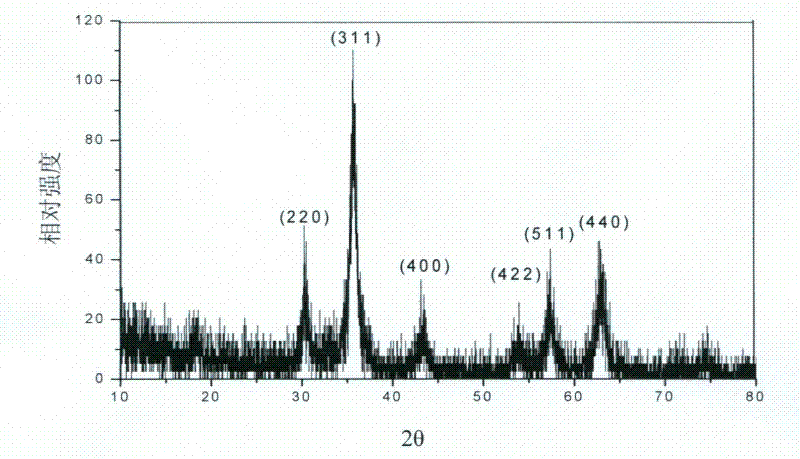
[0029] Characterized by X-ray diffraction technique, it can be seen from the figure that the peaks at 2θ at 30.2, 35.6, 43.2, 53.7, 57.2 and 62.9 correspond to t...
Embodiment 2
[0037] Example 2: Hydroxypropyl-β-cyclodextrin / Fe 3 o 4 Preparation of magnetic nanocomposites
[0038] The first two steps reaction method sees embodiment 1
[0039] (3) Preparation of citric acid modified hydroxypropyl-β-cyclodextrin
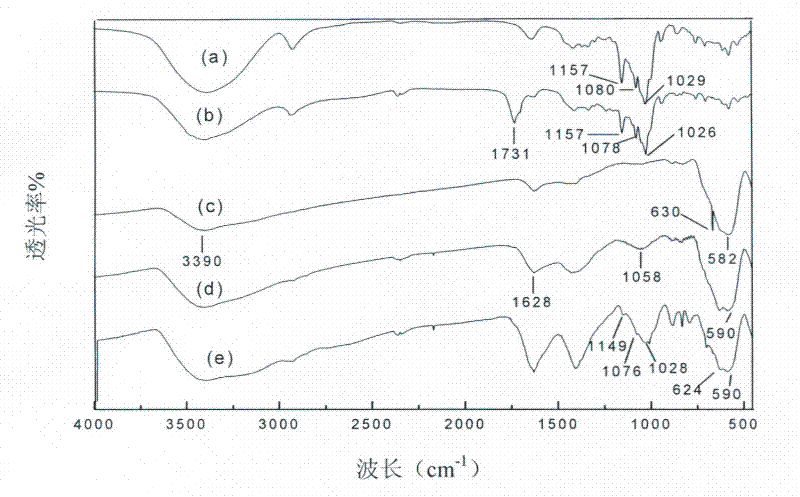
[0040] Weigh 2.5g of hydroxypropyl-β-cyclodextrin, dissolve it in 1.8mL of twice-distilled aqueous solution containing 0.84g of citric acid, react in an oven at 100°C for 3.5 hours, and wash the product with isopropanol. Suction filtration and purification, vacuum drying at 55°C for 10 hours to obtain citric acid-modified hydroxypropyl-β-cyclodextrin (CA-HP-β-CD), which was sealed and stored.
[0041] (4) Hydroxypropyl-β-cyclodextrin / Fe 3 o 4 Preparation of magnetic nanocomposites
[0042] Weigh 1.0 g of GAMNP and dissolve it in buffer solution (0.1 g / mL, phosphate buffer pH=6.0). After ultrasonication for 20 minutes, add 0.25 g of carbodiimide solution (0.025 g / mL), continue ultrasonication for 30 minutes, and finally Add 1.0 g (0.05 g...
Embodiment 3
[0044] Embodiment 3 sulfobutyl ether-β-cyclodextrin / Fe 3 o 4 Preparation of magnetic nanocomposites
[0045] The first two steps reaction method sees embodiment 1
[0046] (3) Preparation of citric acid modified sulfobutyl ether-β-cyclodextrin
[0047] Weigh 2.0 g of sulfobutyl ether-β-cyclodextrin, dissolve it in 1 mL of double-distilled aqueous solution containing 0.68 g of citric acid, react in an oven at 100 ° C for 3.5 hours, wash the product with isopropanol, and pump Purify by filtration, and dry under vacuum at 55°C for 10 hours to obtain citric acid-modified sulfobutyl ether-β-cyclodextrin (CA-SBE-β-CD), which is sealed and stored.
[0048] (4) sulfobutyl ether-β-cyclodextrin / Fe 3 o 4 Preparation of magnetic nanocomposites
[0049] Weigh 1.0 g of GAMNP and dissolve it in buffer solution (0.1 g / mL, phosphate buffer pH=6.0). After ultrasonication for 20 minutes, add 0.25 g of carbodiimide solution (0.025 g / mL), continue ultrasonication for 30 minutes, and finally ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com