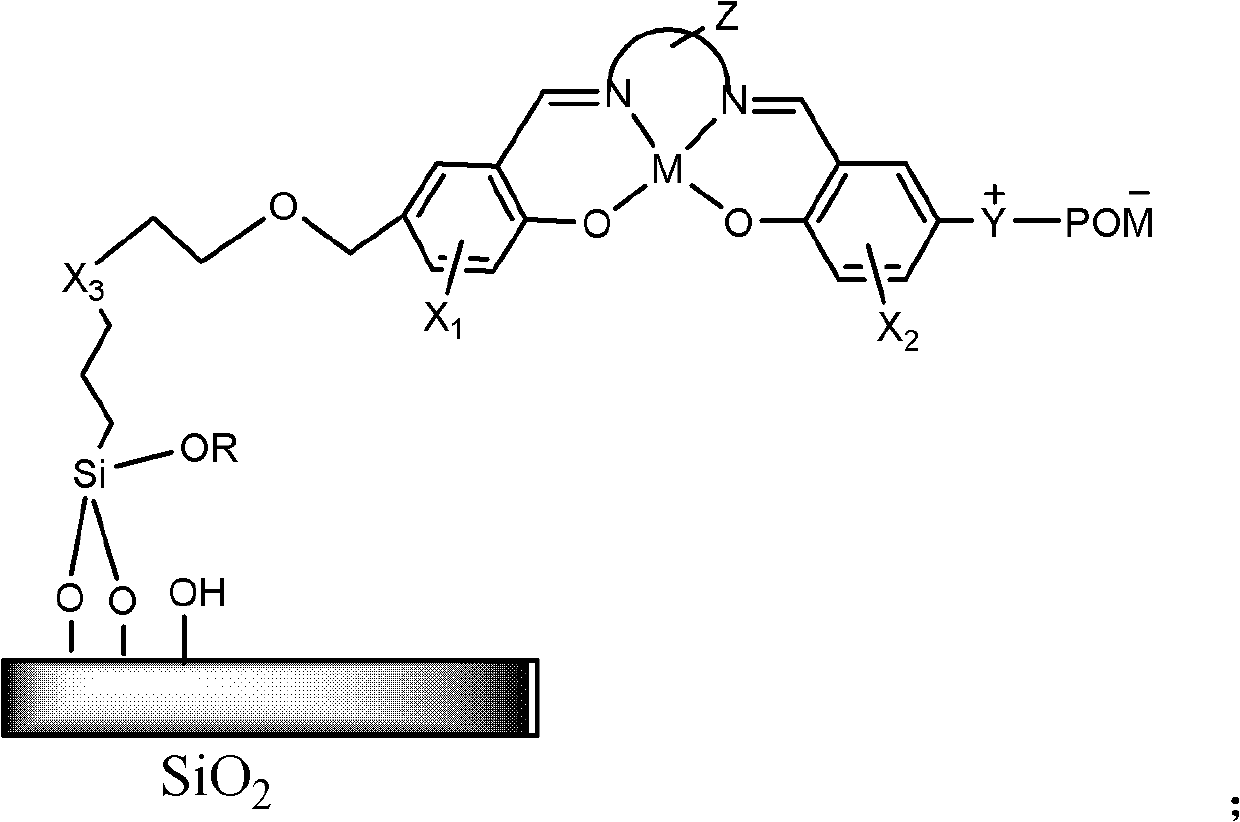
Heterogeneous catalyst for synthesis of phenol, and preparation method and application thereof
A heterogeneous catalyst, phenol technology, applied in the preparation of organic compounds, chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problem of low selectivity, low synthesis yield, use of The problem of short lifespan, etc., can achieve the effect of improving benzene conversion rate and phenol selectivity, good phenol selectivity and high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
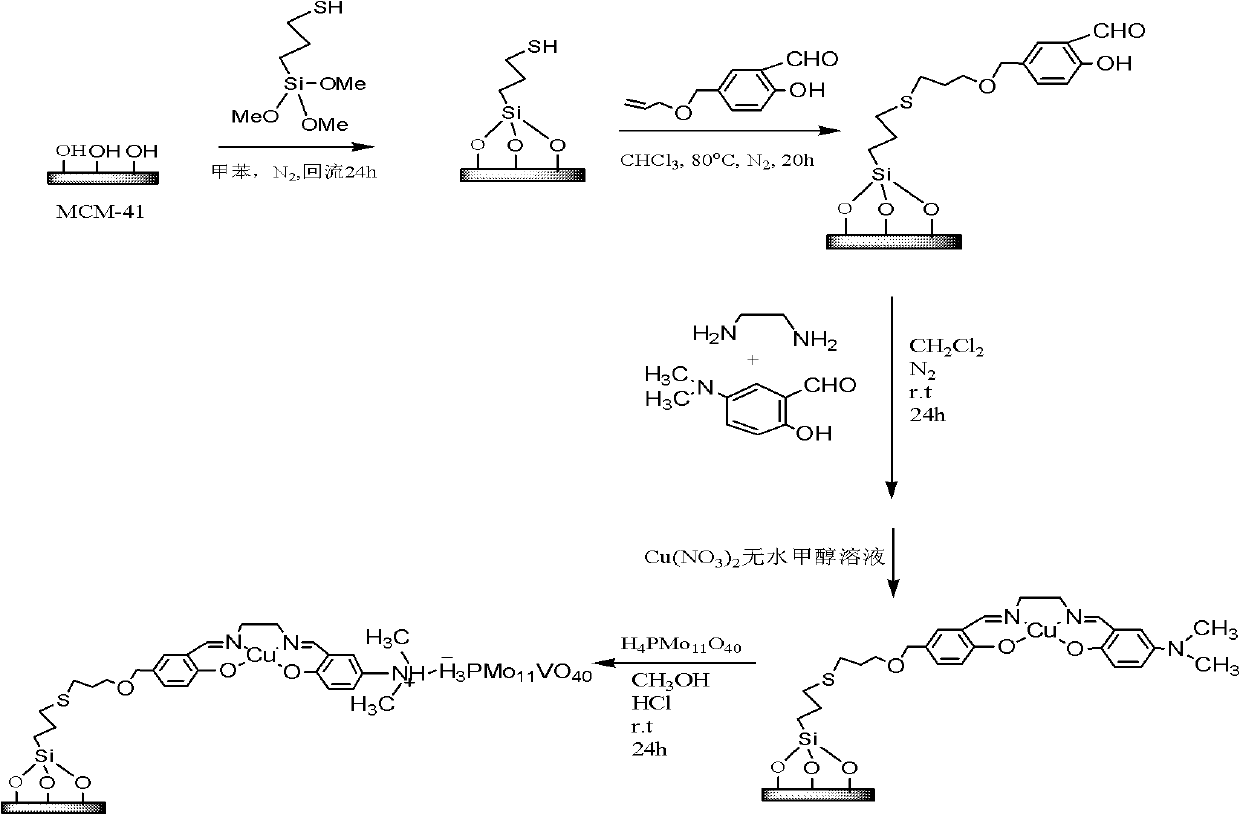
[0029] Example 1: Cu II (salen)-PMo 11 Preparation of V / MCM-41 (salen=disalicylaldehyde ethylenediamine).
[0030] (1) 5-chloromethyl salicylaldehyde was prepared by literature method, and the yield was 40-60%. (Sun Zhenhua et al., Liaoning Chemical Industry, 2010, 39(5)461)
[0031] (2) 5-chloromethyl salicylaldehyde and allyl alcohol were refluxed in xylene for 24 hours in a molar ratio of 1:1 to obtain salicylaldehyde-5-methoxy allyl alcohol ether.
[0032] (3) Mix 1 gram of MCM-41 and 0.016mol 3-mercaptopropyltrimethoxysilane in dry toluene, and the concentration of 3-mercaptopropyltrimethoxysilane in dry toluene is 1.6mol / L. Mixture in N 2 Reflux for 48 hours under protection, filter the solid and perform Soxhlet extraction with dichloromethane for 24 hours to obtain SH-MCM-41. 1gSH-MCM-41, 5mmol salicylaldehyde-5-methoxy allyl alcohol ether, 5mmol 5-dimethylaminosalicylaldehyde, 5mmol ethylenediamine in CH 2 Cl 2 Mix in medium, react for 24h to get the loaded sale...
example 2
[0038] Example 2: Cu II (salen)-PMo 10 V 2 Preparation of / MCM-41 (salen=disalicylaldehyde ethylenediamine).
[0039] Steps (1)-(3) of this example are the same as steps (1)-(3) of Example 1.
[0040] The 4th step is: loading copper salen complex and H 5 PMo 10 V 2 o 40 React in anhydrous methanol acidified with HCl at a molar ratio of 1:1 for 24 h, filter, CH 2 Cl 2 Washed, vacuum dried, heterogeneous catalyst.
[0041] (5) Take the prepared Cu II (salen)-PMo 10 V 2 / MCM-41, benzene, 30%H 2 o 2 1. Add anhydrous acetonitrile into the three-necked flask at a mass ratio of 2:6:14:78, install a condenser tube and a thermometer, and use magnetic stirring. After the temperature rose to 65° C., reacted for 6 hours, filtered the catalyst, collected the filtrate, and analyzed by gas chromatography, the yield of phenol was 14.8%, and the selectivity was 90.5%.
[0042] (6) Take the prepared Cu II (salen)-PMo 10 V 2 / MCM-41 Benzene, Ascorbic Acid, Glacial Acetic Acid, ...
example 3
[0043] Example 3: Cu II (saloph)-PMo 11 Preparation of V / MCM-41 (saloph = salicylaldehyde ortho-phenylenediamine).
[0044] Steps (1)-(2) of this example are the same as steps (1)-(2) of Example 1.
[0045] Step (3) is: mix 1 gram of MCM-41, 0.016mol 3-mercaptopropyltrimethoxysilane in dry toluene, the concentration of 3-mercaptopropyltrimethoxysilane in dry toluene is 1.6mol / L. Mixture in N 2 Reflux for 48 hours under protection, filter the solid and perform Soxhlet extraction with dichloromethane for 24 hours to obtain SH-MCM-41. Mix 1 gram of SH-MCM-41 and 5 mmol of salicylaldehyde-5-methoxy allyl alcohol ether in xylene, and 1 gram of dried solid contains about 3 mmol of salicylaldehyde. Then add and dry the CH of 5-dimethylamino salicylaldehyde and o-phenylenediamine equimolar salicylaldehyde contained in the dry solid 2 Cl 2 The solution was stirred and reacted at room temperature for 24 hours to obtain the loaded saloph ligand, and the thermogravimetric results ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com