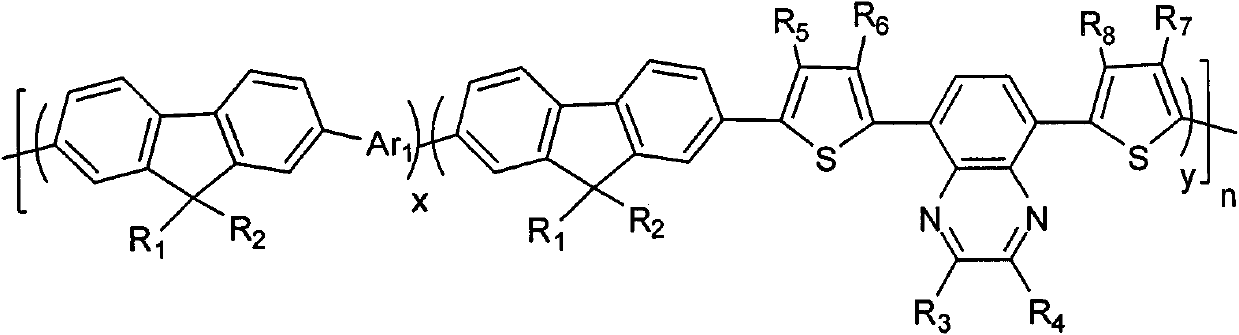
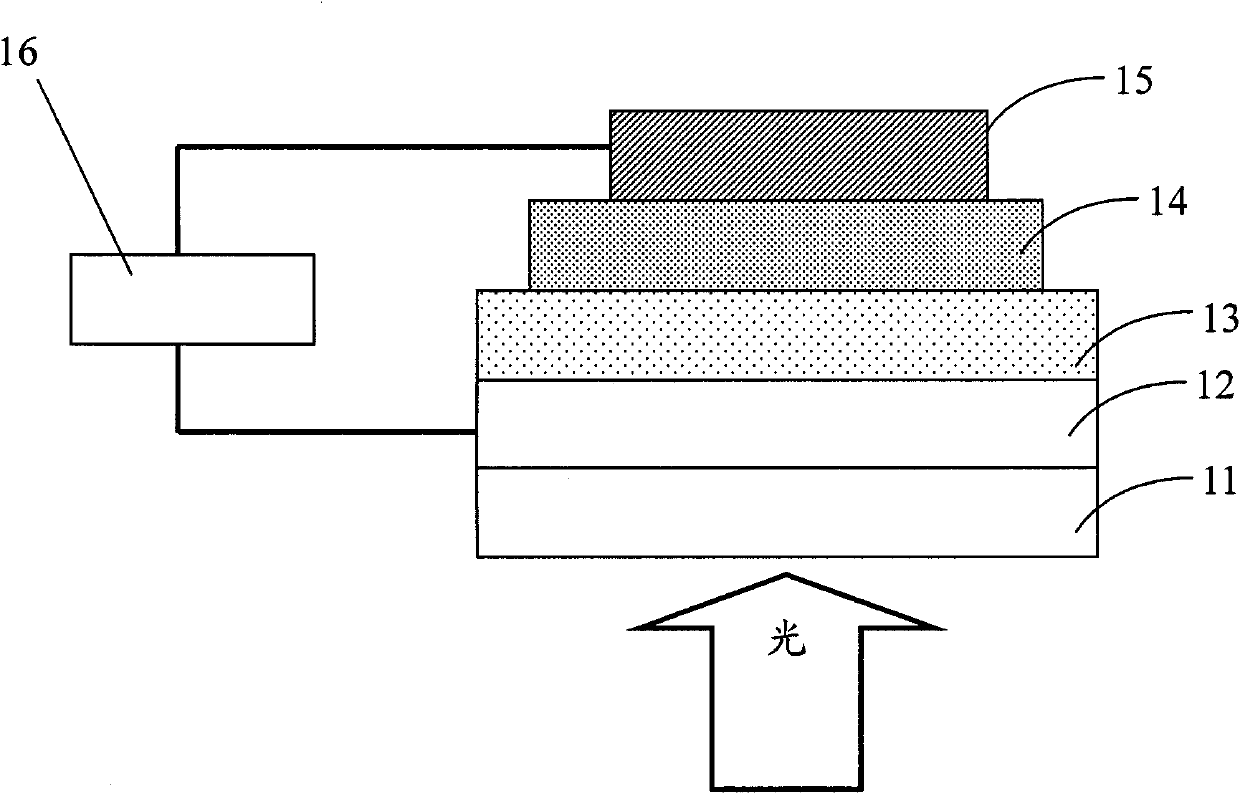
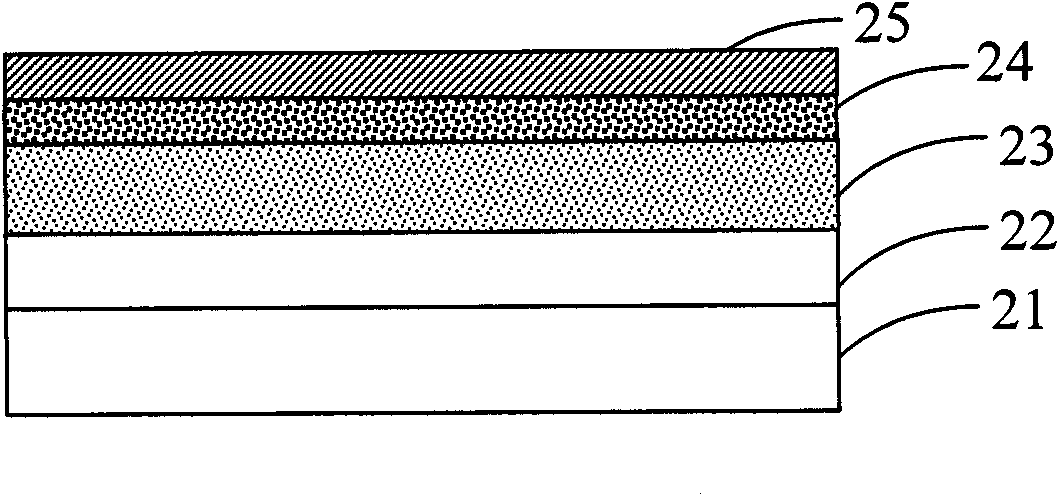
Thiophene-quinoxaline unit containing fluorene copolymer as well as preparation method and application thereof
A kind of copolymer, quinoxaline technology, applied in the field of fluorene copolymer containing thiophene-quinoxaline unit and its preparation, can solve the problem of low photoelectric conversion efficiency, achieve improved photoelectric conversion efficiency, wide spectral response range, easy Effects of manipulation and control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] The preferred scheme of the preparation method of the above-mentioned compound A is: in an anhydrous, oxygen-free environment and an organic solvent system, first react 2,7-dibromo-9,9-dialkylfluorene with an alkyl derivative of lithium , and then add 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane for reaction to generate the compound A.
[0055] In the preparation steps of the above-mentioned compound A, the 2,7-dibromo-9,9-dialkylfluorene, the alkyl derivative of lithium and 2-isopropoxy-4,4,5,5-tetramethyl The molar ratio of base-1,3,2-dioxaborolane three reactants is preferably 1:2.0~4.0:2.0~4.0; the alkyl derivative of lithium is preferably n-butyllithium, tert-butyllithium, At least one of methyllithium and phenyllithium; the organic solvent is preferably at least one of tetrahydrofuran, ether, methylene chloride, chloroform or ethyl acetate; the reaction temperature of the preparation step of compound A is preferably -100 ~-25°C, the time is preferably 24-...
Embodiment 1
[0075]Containing thiophene-quinoxaline unit fluorene copolymer I of the present embodiment 1 The structural formula is as follows:
[0076]
[0077] Its preparation steps are as follows:
[0078] (1) Preparation of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolyl)-9,9-dimethylfluorene, whose structural formula is as follows :
[0079]
[0080] The specific preparation process is as follows: at -100°C and under nitrogen, add 20.00 mL of n-butyllithium oil solution with a concentration of 1.00 mol / L to 3.52 g of 2,7-dibromo-9,9-dimethyl fluorene and 100mL tetrahydrofuran reaction flask, after stirring for 2 hours, slowly add 4.17mL of 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxa Pentaborane, returned to room temperature, continued to stir for 24 hours, after the reaction was completed, the reaction solution was poured into water, extracted with ether, dried over anhydrous magnesium sulfate, rotary evaporated, and separated by column chromatography to obtain a solid produc...
Embodiment 2
[0094] Fluorene Copolymers Containing Thiophene-Quinoxaline Units I 2 The preparation, its structural formula is as follows:
[0095]
[0096] Its preparation steps are as follows:
[0097] (1) Preparation of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolyl)-9,9-dimethylfluorene: detailed preparation steps See embodiment 1, its structural formula is as follows:
[0098]
[0099] (2) The preparation of 3,6-dimethylthieno[3,2-b]thiophene, its structural formula is as follows:
[0100]
[0101] The specific process of preparation is: 12.00g of 3,6-dibromo-thieno[3,2-b]thiophene and 132mg (1,1'-bis(diphenylphosphino)ferrocene)palladium chloride (II) Add it to a 100mL tubular glass bottle equipped with a stirring bar, seal it, blow it with nitrogen, add 30mL tetrahydrofuran and 50mL methyl zinc bromide (dissolve methyl zinc bromide in tetrahydrofuran solution, its concentration is 1.0 mol / L), stirred at room temperature for 30 minutes, heated in a microwave reactor at 14...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com