A method for enzymatic protein cyclization
An enzyme protein, cyclase protein technology, applied in biochemical equipment and methods, microorganism-based methods, botanical equipment and methods, etc., can solve problems such as unfavorable in vitro cyclization, and achieve the effect of good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
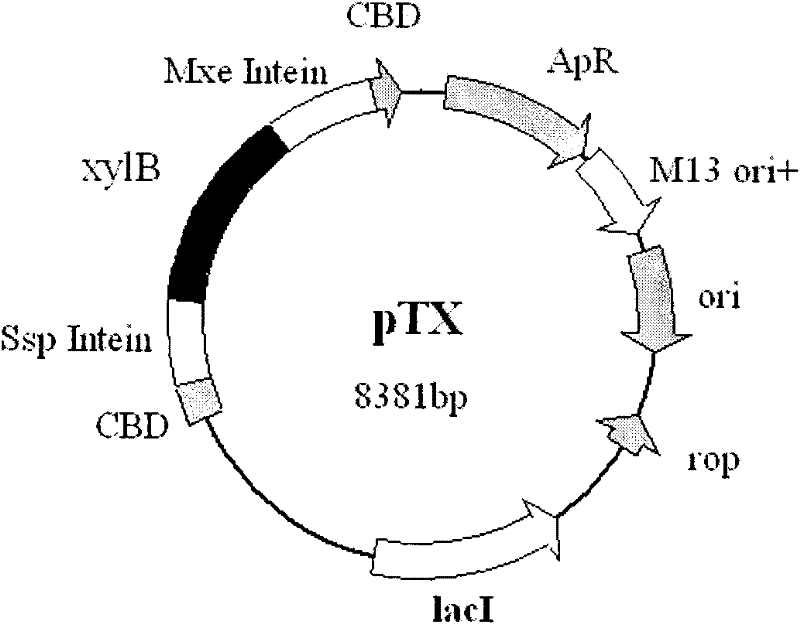
[0030] Cloning of embodiment 1 xylanase gene and construction of circularization vector
[0031] Firstly, the plasmid pTWIN1 (ENB Company) was extracted, and the sequence of the Synechocystis Ssp DnaB intein was amplified by PCR using the extracted plasmid as a template. The primers used were synthesized according to the 5' and 3' end sequences of the DnaB gene. The sequences of the two primers were: 5'ATACATATGA AAATCGAAGA AGGTAAAC 3' and 5'GAATTCGTTG TGTACAATGA TGTCATTC 3'. After amplification, gel electrophoresis was performed to recover the size 680bp fragments. Afterwards, the fragments were recovered after Nde I and EcoRI double digestion. In addition, the sequence of the Mxe GyrA intein of Mycobacterium xenopus was amplified by PCR using the proposed plasmid as a template. The primers used were synthesized according to the 5' and 3' end sequences of the Mxe GyrA gene. The sequences of the two primers were: 5'CTCGAGTGCATCACGGGAGATGCACTAG 3' and 5'GGATCCCCTTCCTGCAGTCATT...
Embodiment 2
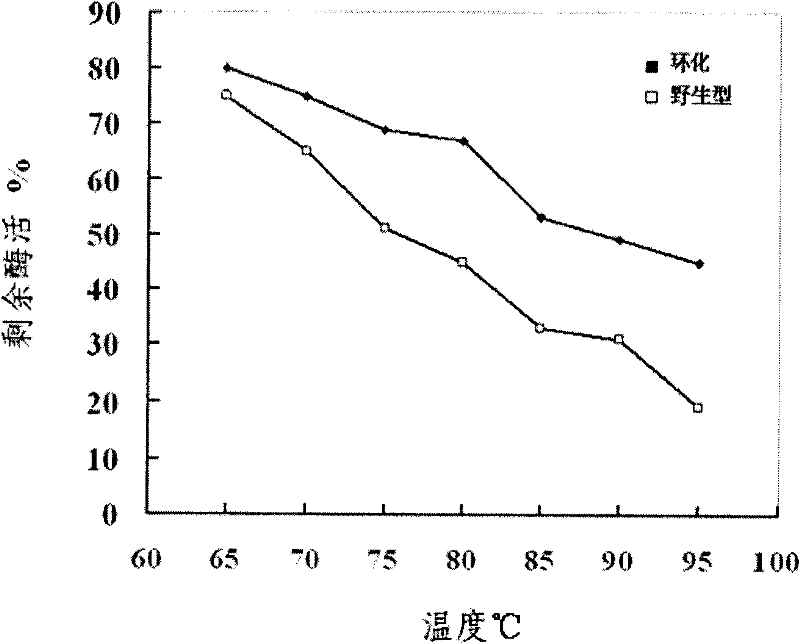
[0033] The in vitro cyclization of embodiment 2 xylanase
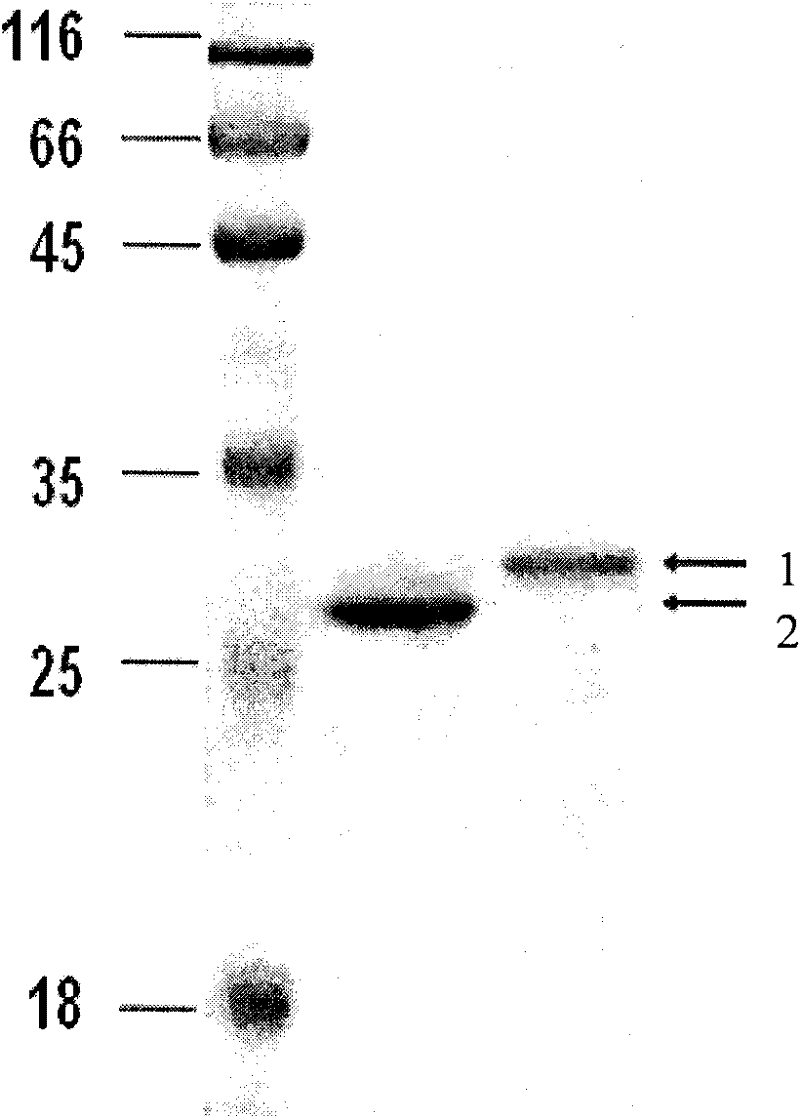
[0034] 1) Protein cyclization and purification procedures
[0035]Inoculate the recombinants into 20 mL of LB medium, culture with shaking at 37°C overnight, inoculate in 1 liter of LB medium at 1% inoculum size, culture at 37°C until OD is 0.5, add IPTG to a final concentration of 0.5mM, continue After culturing for 3 hours, the expression of xylanase was induced. The bacteria were collected by centrifugation, and the bacteria were resuspended in 100 mL of Tris-HCl (pH 7.0) buffer containing 500 mM NaCl, disrupted by ultrasonic waves, and centrifuged at 13000 rpm for 30 minutes to remove cell debris. The supernatant was passed through a chitin column, and then the column was thoroughly washed with 20 column bed volumes of buffer 1 (20 mM Tris-HCl pH 7.0, 0.5 M NaCl). Stop the column flow and place at 20°C for 16 hours to induce cleavage of the Ssp DnaB intein. Afterwards, pass through the column with buffer solutio...
Embodiment 3
[0038] Embodiment 3 cycloxylanase activity assay
[0039] 1) Standard curve creation
[0040] Draw 1% xylose standard solution 1.0, 2.0, 3.0, 4.0, 5.0ml into a 50ml volumetric flask, distill the volume to the mark with distilled water, dilute to 0.2, 0.4, 0.6, 0.8, 1.0mg / ml xylose standard liquid. Take 0.5ml of diluted xylose standard solution of different concentrations in three test tubes, add 1.5ml of sodium dihydrogen phosphate-citric acid buffer, add 3.0ml of DNS reagent, bathe in boiling water for 5 minutes, then dilute 10ml with deionized water, Mix well. Deionized water was used as a control. Absorbance was measured at 540 nm after cooling. Draw the standard curve with the absorbance value as the ordinate and the xylose concentration as the abscissa. The regression equation of the xylose standard curve is y=0.148x-0.0754, where R 2 = 0.9997.
[0041] 2) Determination of xylanase activity
[0042] Enzyme activity unit (U) is defined as: the amount of enzyme that...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com