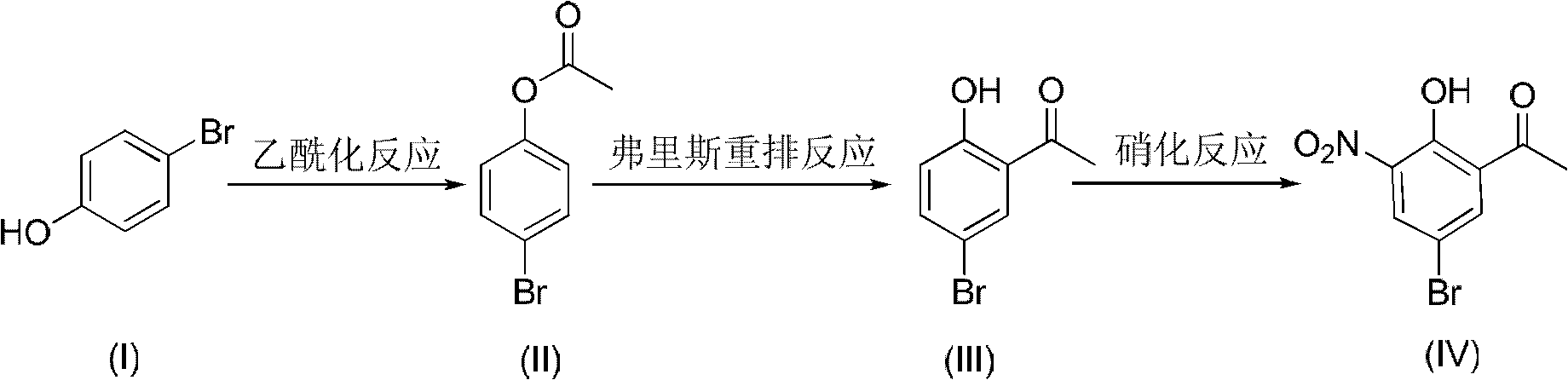
Method for preparing 5-bromo-2-hydroxy-3-nitroacetophenone
A technology of nitroacetophenone and hydroxyl, which is applied in the field of preparation of pharmaceutical intermediates, can solve the problems of large amount of solvent, high risk, cumbersome reaction operation, etc., so as to reduce the amount of solvent, shorten the post-processing time, and simplify the process operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 173g of p-bromophenol (1mol) was dissolved in 690ml of tetrachlorethylene, 138g of potassium carbonate (1mol) was added, the temperature was controlled at 5°C, 107g of acetic anhydride (1.04mol) was slowly added, reacted for 2.5 hours, and the concentration of 600ml was slowly added to the system The hydrochloric acid of 3.5mol / L was stirred for 10 minutes, left to stand for layering, and extracted to obtain the light yellow tetrachloroethylene liquid layer of the lower floor; the light yellow tetrachloroethylene solution was transferred to another reaction flask, and 160g anhydrous aluminum chloride ( 1.2mol), reflux at 110°C for 7 hours, stop heating, slowly add 500ml of hydrochloric acid with a concentration of 3mol / L to the system when the temperature is lowered to 80°C, stir at room temperature for 1 hour, let stand to separate layers, and extract the lower layer of dark brown tetrachloride Ethylene liquid layer; transfer the dark brown tetrachlorethylene solution t...
Embodiment 2
[0036] 173g of p-bromophenol (1mol) was dissolved in 520ml of tetrachlorethylene, 95g of pyridine (1.2mol) was added, the temperature was controlled at 0°C, 90.3g of acetyl chloride (1.15mol) was slowly added, reacted for 1.5 hours, and the concentration of 390ml was added to the system 2mol / L of sulfuric acid, stirred for 30 minutes, left to stand for layering, and extracted to obtain a light yellow tetrachlorethylene liquid layer in the lower floor; the light yellow tetrachlorethylene solution was transferred to another reaction flask, and 173g of anhydrous aluminum chloride (1.3 mol), reflux at 121°C for 5.5 hours, stop heating, and slowly add 500ml of hydrochloric acid with a concentration of 3mol / L to the system when the temperature is lowered to 80°C, stir at room temperature for 1 hour, let stand to separate layers, and extract the lower layer of dark brown tetrachloroethylene Liquid layer; transfer the dark brown tetrachlorethylene solution to another reaction flask, sl...
Embodiment 3
[0038] Dissolve 200g of p-bromophenol (1.16mol) in 540ml of tetrachlorethylene, add 129g of triethylamine (1.28mol), control the temperature at 0°C, slowly add 130g of acetic anhydride (1.27mol), react for 2 hours, add 400ml of phosphoric acid with a concentration of 1mol / L, stirred for 30 minutes, allowed to stand for stratification, and extracted to obtain a light yellow tetrachloroethylene liquid layer in the lower layer; transfer the light yellow tetrachloroethylene solution to another reaction bottle, and add 208g of anhydrous chlorinated Aluminum (1.56mol), reflux at 121°C for 6 hours, stop heating, slowly add 600ml of phosphoric acid with a concentration of 1.5mol / L to the system when the temperature drops to 85°C, stir at room temperature for 40 minutes, let stand to separate layers, and extract to obtain the lower layer deep Brown tetrachlorethylene liquid layer; transfer the dark brown tetrachlorethylene solution to another reaction flask, slowly add 101g of nitric ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com