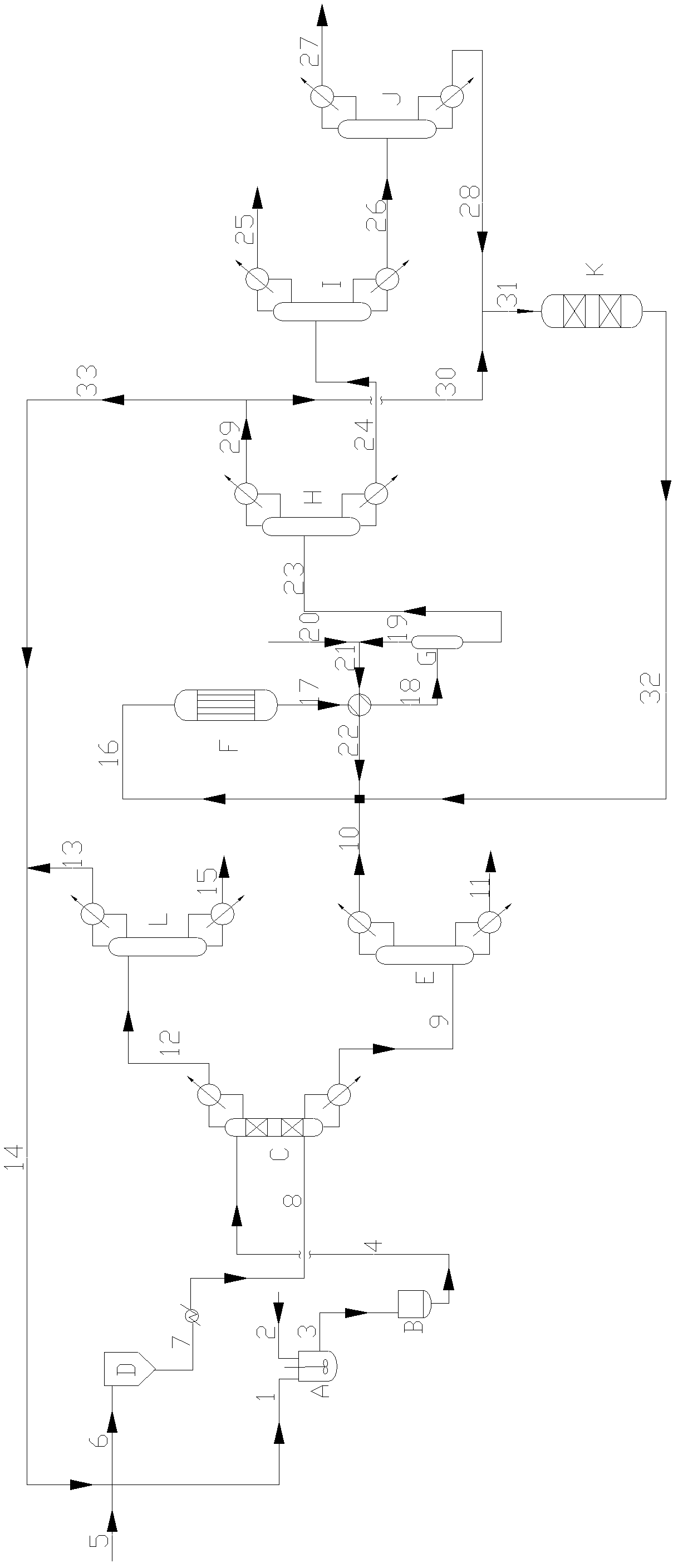Process for producing 1,6-hexanediol and coproducing epsilon-caprolactone
A technology of hexanediol and caprolactone, which is applied in the field of adipate esterification hydrogenation preparation 1, can solve the problems of difficult separation of products and catalysts, high energy consumption for product separation and purification, and a large amount of acidic wastewater, so as to save separation energy consumption, save equipment investment, and shorten the production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
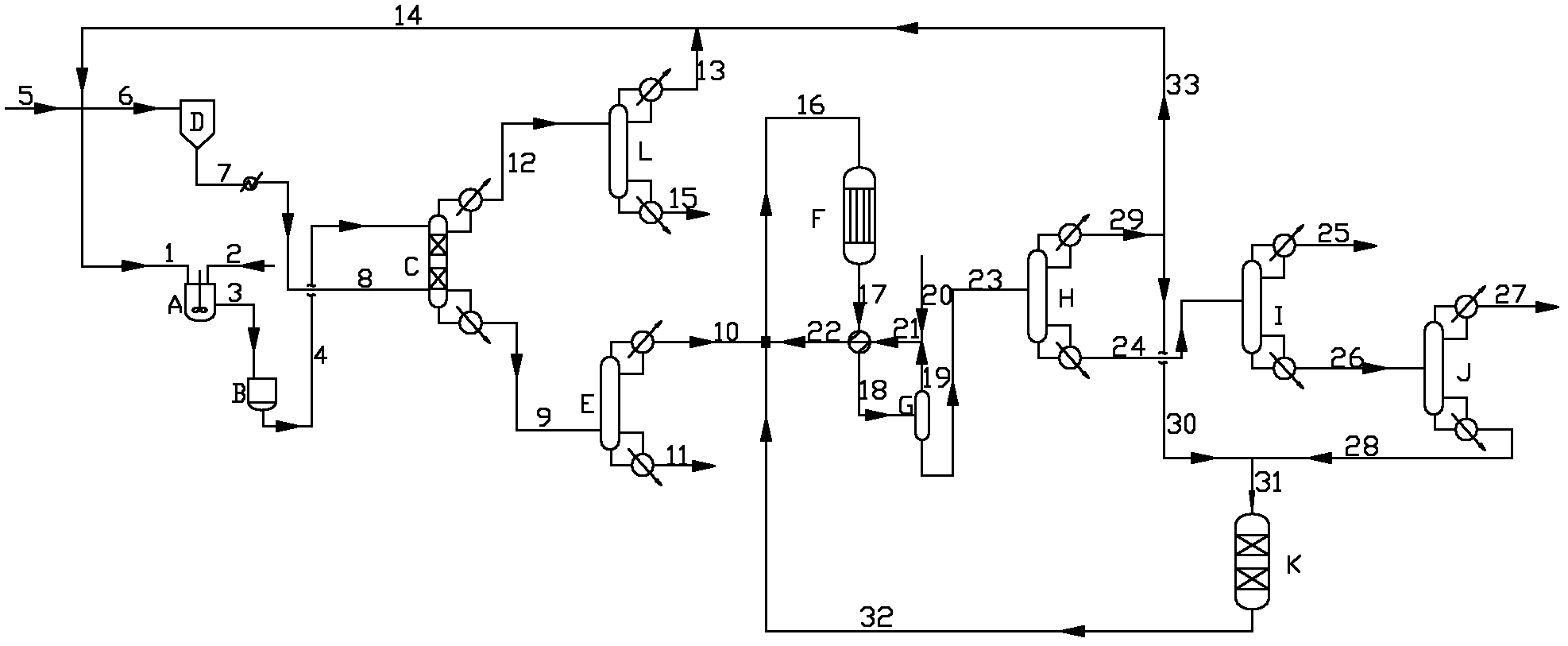
[0046] In this example, refer to the attached figure 1 The apparatus and procedures shown set up the various reaction components.
[0047] In the reactive distillation column C (inner diameter 40mm, the pipe of height 4000mm), fill the filler of 50 theoretical plate heights, in the tower, filler particle average loading superacid active center 0.6% (weight ratio), filler particle average size is 5mm×5mm.
[0048] This filler is a self-made filler, first of all (NH4) 2 S 2 o 8 Immerse it on the nano-beta molecular sieve to make catalyst nano-scale powder (particle size is 68-80nm), adopt the mode of spraying or mechanical mixing, preferably mechanical mixing mode, and mix described catalyst nano-scale powder with ceramic mud and binder ( Hydroxypropyl methylcellulose and / or squash powder), dried, and roasted at 550°C for 4 hours, and its size is 3-5mm×3-5mm, preferably 5mm×5mm.
[0049] The adipic acid liquid passes through the first tray at the top of the reactive distill...
Embodiment 2
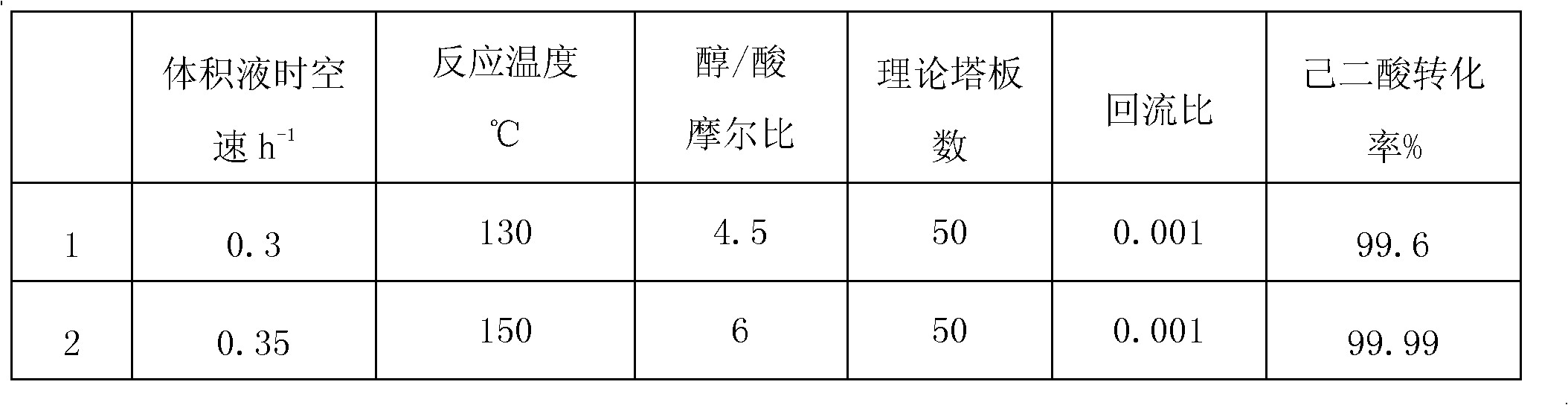
[0056] Adipic acid and methanol are continuously esterified in a reactive distillation tower, and the process flow is the same as in Example 1. The reaction pressure is normal pressure, the reaction temperature is 100-150°C, and the liquid hourly space velocity is 0.8Kg / Kg.h. The specific results are as follows:
[0057] Table 1
[0058]
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
| Height | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com