Heat-resistant cutinase-CBD (cellulose-binding domain) fusion enzyme, its mutants and application
A cutinase, mutant technology, applied in the field of genetic engineering and enzyme engineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
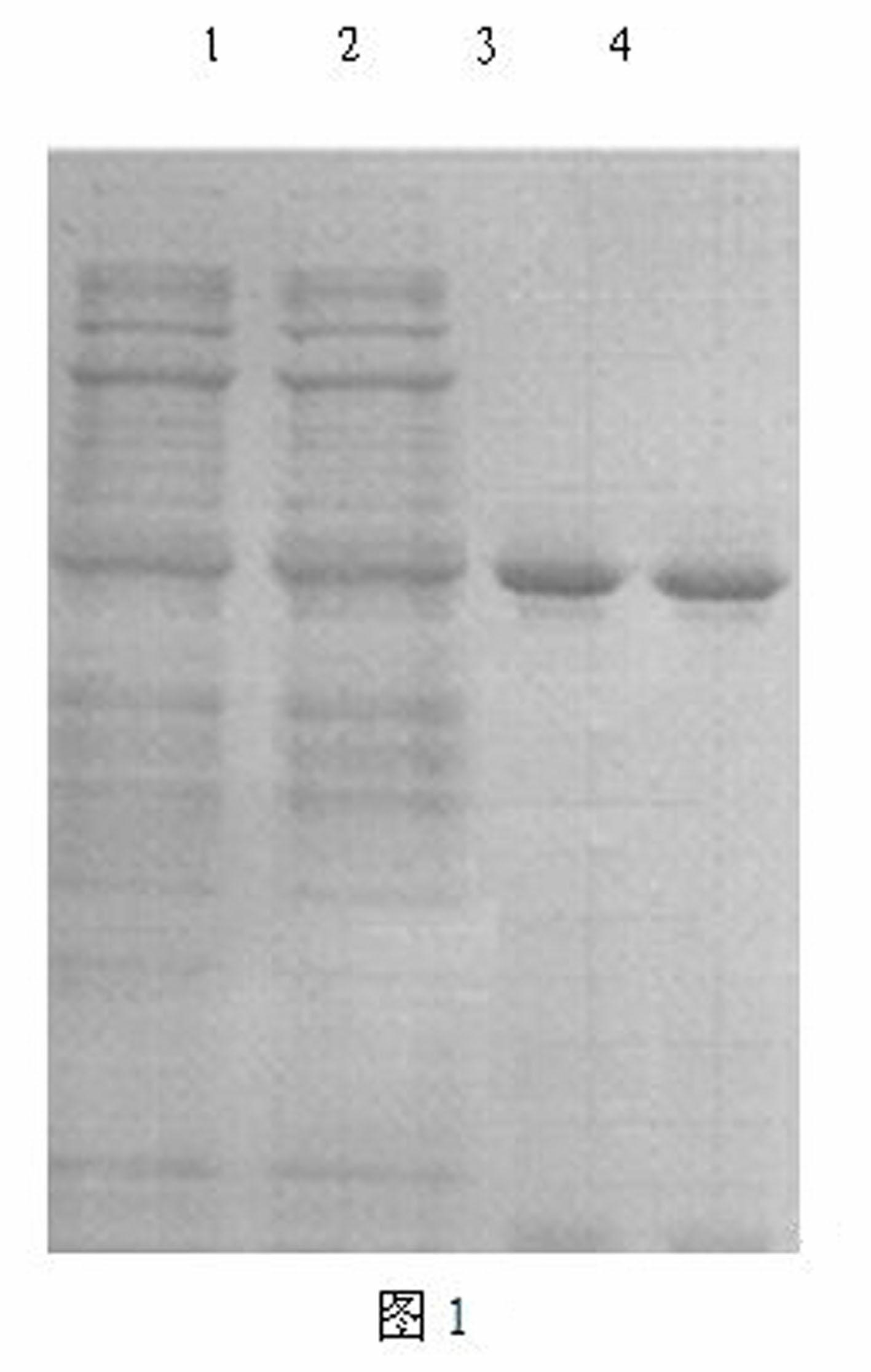
[0016] Embodiment 1 The preparation method of cutinase-CBD fusion enzyme
[0017] The fusion gene encoding cutinase-CBD is obtained by the method of total gene synthesis, and the sequence is as SEQ ID NO: 1. The expression vector of cutinase-CBD gene is plasmid pET20b(+), with E. coli BL21(DE3) was used as the expression host to achieve high-efficiency expression of the thermostable cutinase-CBD gene. Its fermentation and purification methods are the same as those of the cutinase-CBD mutant.
Embodiment 2
[0018] Example 2 Effect of cutinase-CBD fusion enzyme on cotton fiber
[0019] In the 10 mL reaction system, add 1 mL cutinase-CBD (or natural cutinase) enzyme solution (enzyme activity 100 U / mL), 1 mL pectin enzyme solution (enzyme activity 100 U / mL), 8 mL 25 mM potassium phosphate (pH 8.0), 0.5g raw cotton fiber and 50μL penetrant TX-10. The control was the same as the above conditions but no cotton fiber was added to the treatment solution. React at room temperature and take samples regularly. After the sample was centrifuged for 3 min, the enzyme activity in the supernatant was measured according to the method for measuring enzyme activity. The amount of enzyme adsorbed is the enzyme activity in the control solution minus the enzyme activity in the supernatant. The results showed that under the interaction with pectinase, the adsorption rate of natural cutinase to cotton fiber was 10%, while the adsorption rate of cutinase-CBD to cotton fiber was 55%.
[0020] The 10...
Embodiment 3
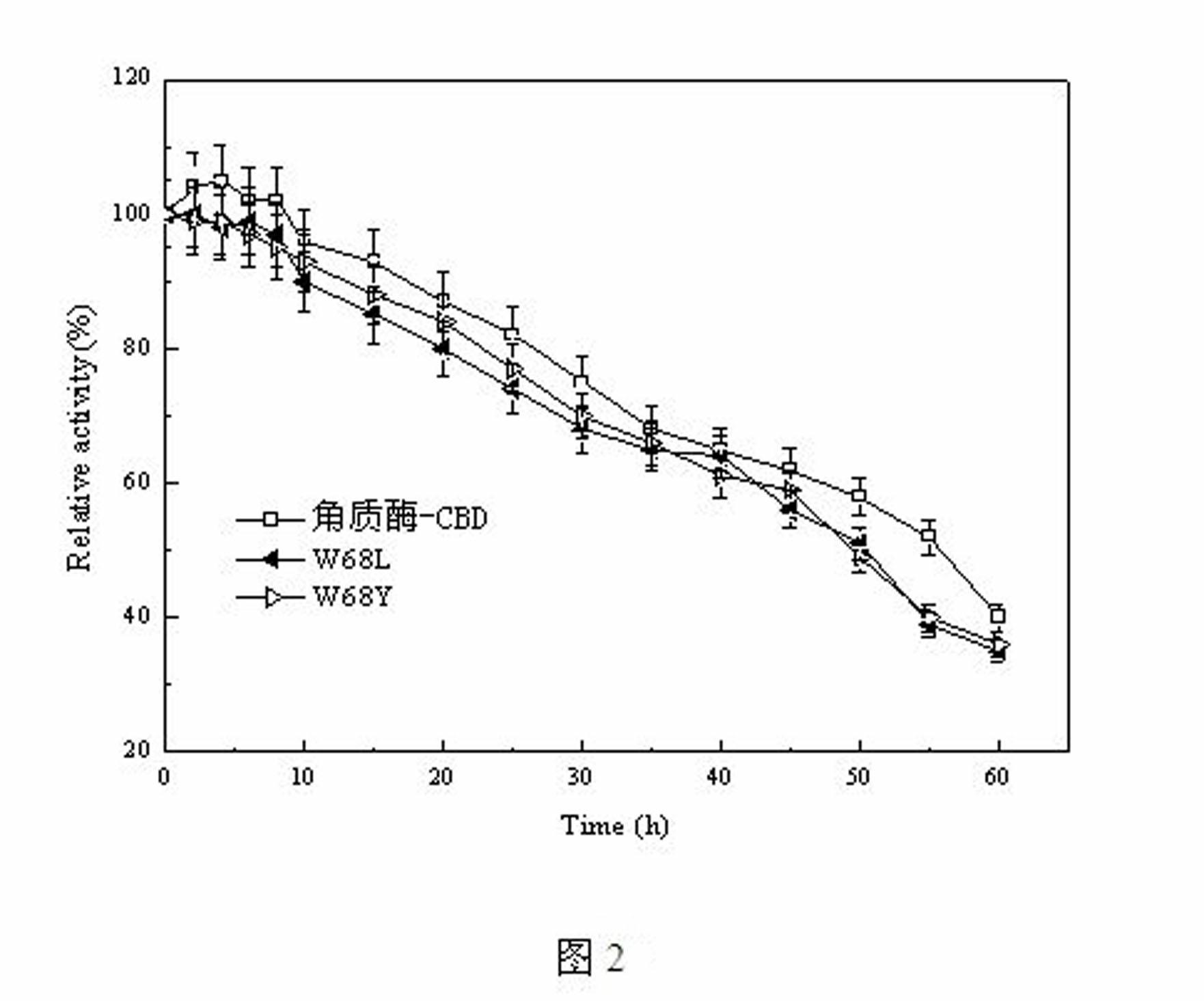
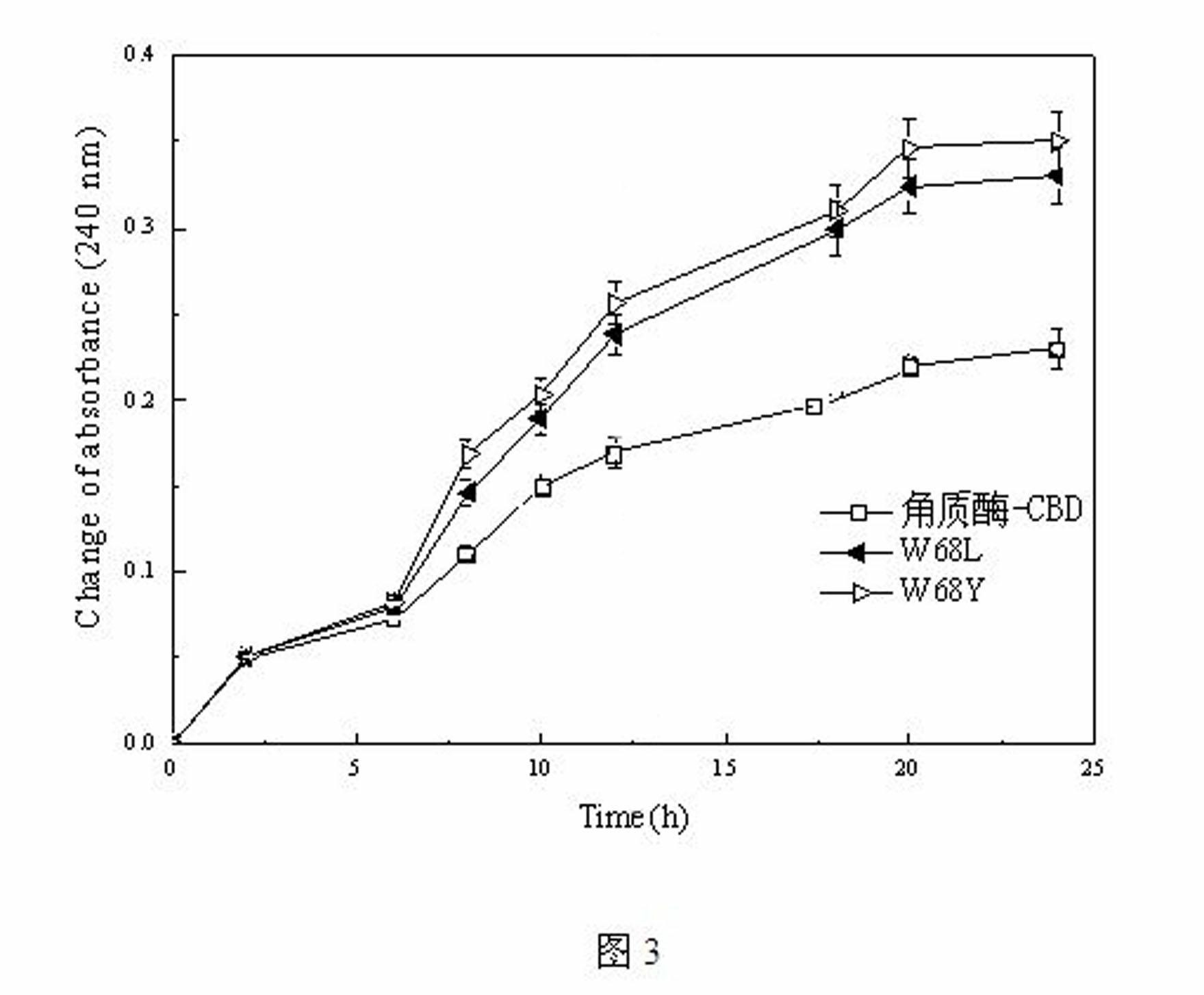
[0021] Example 3 Determination of cutinase-CBD mutant mutation site
[0022] The key amino acid for the cellulose binding domain to bind to cellulose is the highly conserved tryptophan residue. Through the multiple sequence alignment of the second family CBD, combined with the structural characteristics of polyester fibers, two key points were mutated into amino acids similar to polyester polyester structures from the above two aspects to enhance the hydrophobic interaction between the enzyme and the substrate, thereby Improve catalytic efficiency. The specific mutations are as follows: (1) mutate tryptophan to leucine to enhance the hydrophobic interaction with the alkyl chain in the polyester structure to improve the affinity with the substrate; (2) mutate tryptophan to tyrosine Amino acid improves the hydrophobic interaction with the benzene ring in the polyester structure, and the hydroxyl group generates hydrogen bonds to enhance the binding force with the substrate. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com