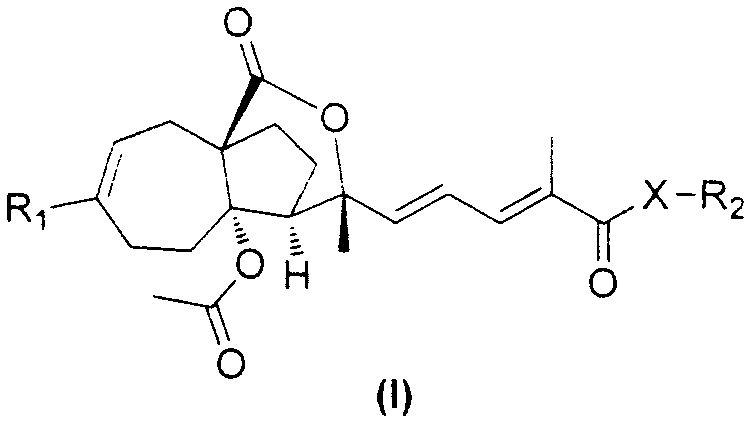
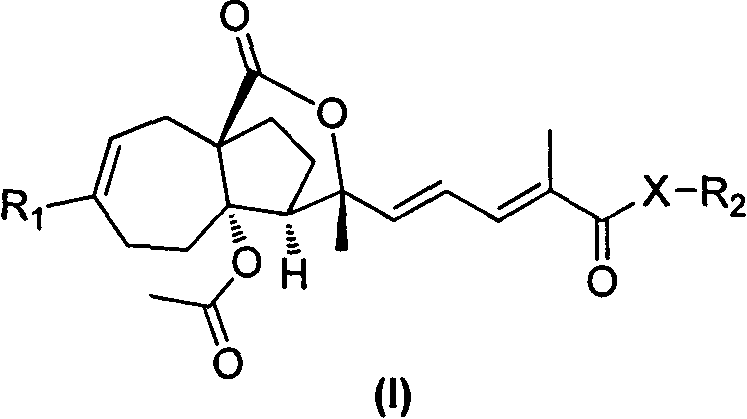
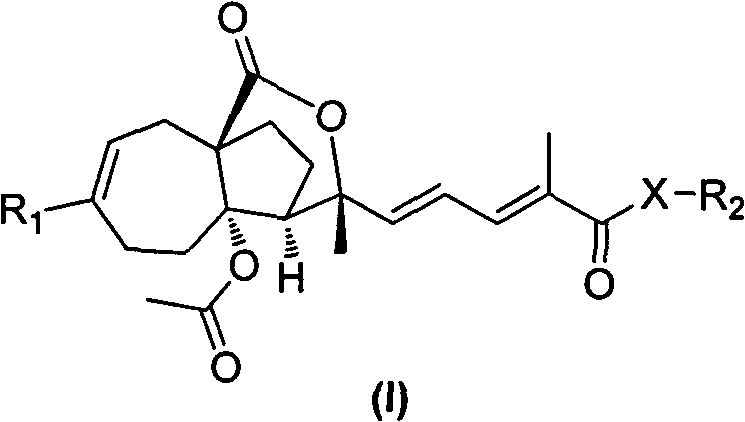
Pseudolarix acid derivatives as well as preparation method and application thereof
A technology of hibiscus bark and its derivatives, applied in the field of medicine, can solve the problems of low bioavailability and poor water solubility, and achieve the effects of stable chemical structure, improved water solubility, and broad-spectrum anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Synthesis of SYN-21
[0043] step 1:
[0044] In a 50ml reaction bottle, add 150mg (0.35mmol) of hibiscus bark acetic acid and 2ml of anhydrous dichloromethane, stir to dissolve, then add 4ml of thionyl chloride dropwise at room temperature, install a reflux condenser and CaCl 2 Dry the tube, reflux for 4 hours, evaporate excess thionyl chloride and solvent under reduced pressure, and the residue is dissolved with 2ml of anhydrous dichloromethane to make solution A.
[0045] Step 2:
[0046] In another 50ml reaction bottle, add 410mg (3.5mmol) of N,N-diethylethanolamine and 2ml of anhydrous dichloromethane and stir, then add solution A drop by drop at room temperature, install a drying tube, and stir at room temperature Reacted for 2 hours, detected by TCL, showed that the reaction was complete, and evaporated the low boilers on the rotary evaporator, and the residue was distributed with ethyl acetate and water, and first used NaHCO 3 The solution was was...
Embodiment 2
[0048] Example 2: Synthesis of SYN-22
[0049] At room temperature, add 3ml dimethylformamide, 110mg (0.25mmol) hibiscus bark acetic acid, 69mg (0.5mmol) K 2 CO 3 , 409 mg (2.5 mmol) 4-(3-chloropropyl) morpholine and 0.5 mg of potassium iodide and stirred, loaded with CaCl 2 Dry the tube, raise the temperature to 50°C overnight, and use TCL to detect that the reaction is complete. Pour it into 50 mL of ethyl acetate, wash 20 mL*5 times with saturated brine, and evaporate the ethyl acetate to dryness to obtain the title compound (SYN-22). Crude. The crude product was subjected to column chromatography on a silica gel column with petroleum ether: ethyl acetate: formic acid = 3:1:0.1, and 120.3 mg of the title compound (SYN-22) was isolated (86.1% yield).
[0050] SYN-22: 1 H NMR (CDCl 3 )δ (ppm): 7.21 (m, 1H), 7.15 (1H, d, J = 11.4Hz), 6.54 (1H, dd, J = 15.1, 11.4Hz), 5.88 (1H, d, J = 15.1Hz) , 4.22(2H, t, J=6.5Hz), 3.72(3H, s), 3.71(4H, m), 3.30(1H, d, J=5.0Hz), 3.09(1H, ...
Embodiment 3
[0051] Example 3: Synthesis of SYN-23
[0052] At room temperature, add 110mg (0.25mmol) hibiscus bark acetic acid, 4ml dimethylformamide, 80mg (0.50mmol) of 2-(2-diethylaminoethoxy) ethanol, triphenyl Phosphorus 131mg (0.50mmol), stir to dissolve, slowly add 101mg (0.50mmol) of diisopropyl azodicarboxylate dropwise, connect calcium chloride drying tube, stir at room temperature for 3 hours, use TCL detection to show that the reaction is complete, pour into 50mL In ethyl acetate, wash 20 mL*5 times with saturated brine, and evaporate the ethyl acetate phase to dryness to obtain the crude product of the title compound. The crude product was subjected to column chromatography on a silica gel column, and 122 mg of the title compound was obtained with a yield of 85%.
[0053] SYN-23: 1 H NMR (CDCl 3 )δ (ppm): 7.21 (m, 1H), 7.18 (1H, d, J = 11.4Hz), 6.54 (1H, dd, J = 15.1, 11.4Hz), 5.87 (1H, d, J = 15.1Hz) , 4.30(2H, t, J=4.9Hz), 3.71(3H, s), 3.72(2H, overlapped), 3.59(2H, t, J...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com