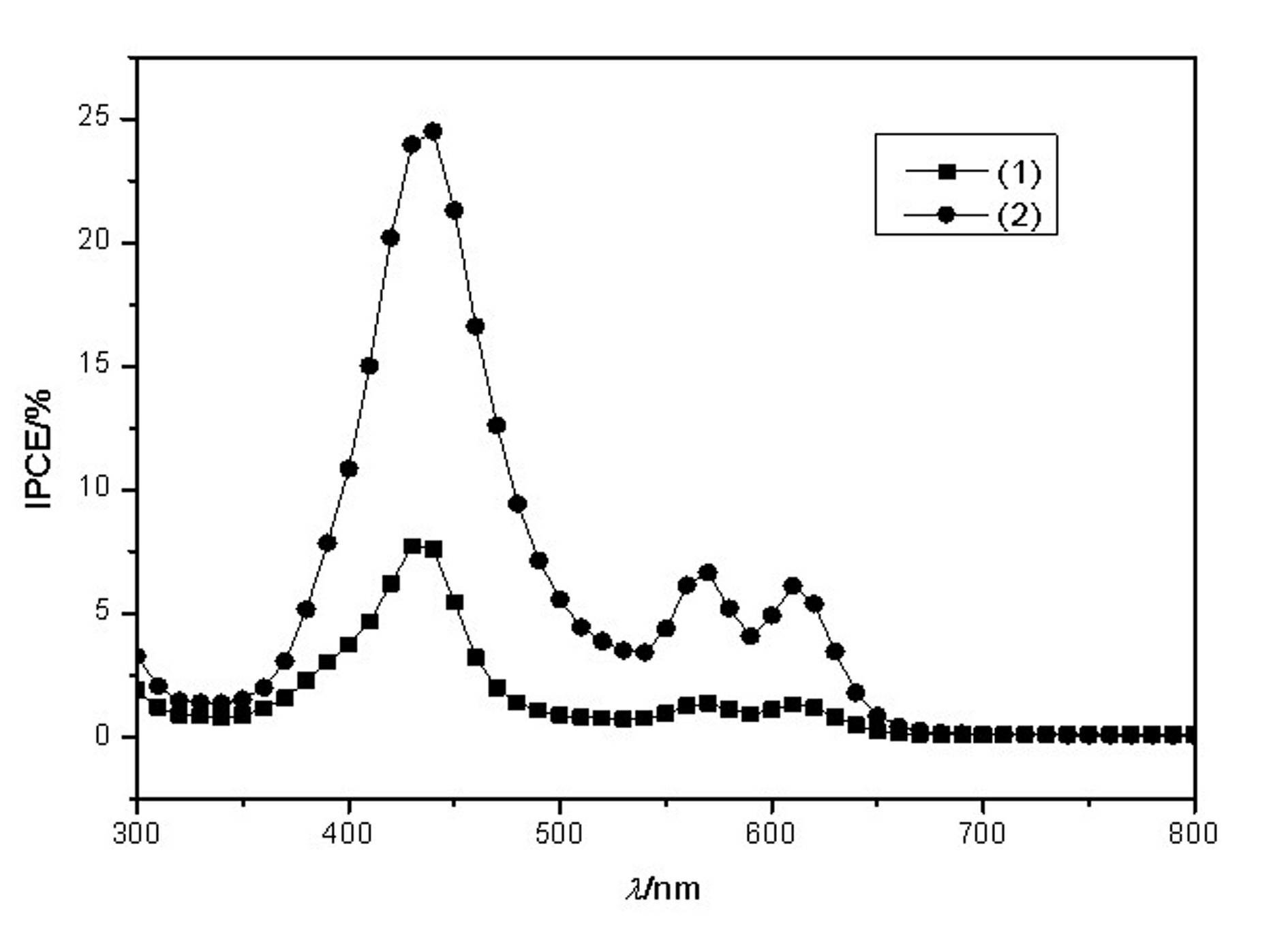
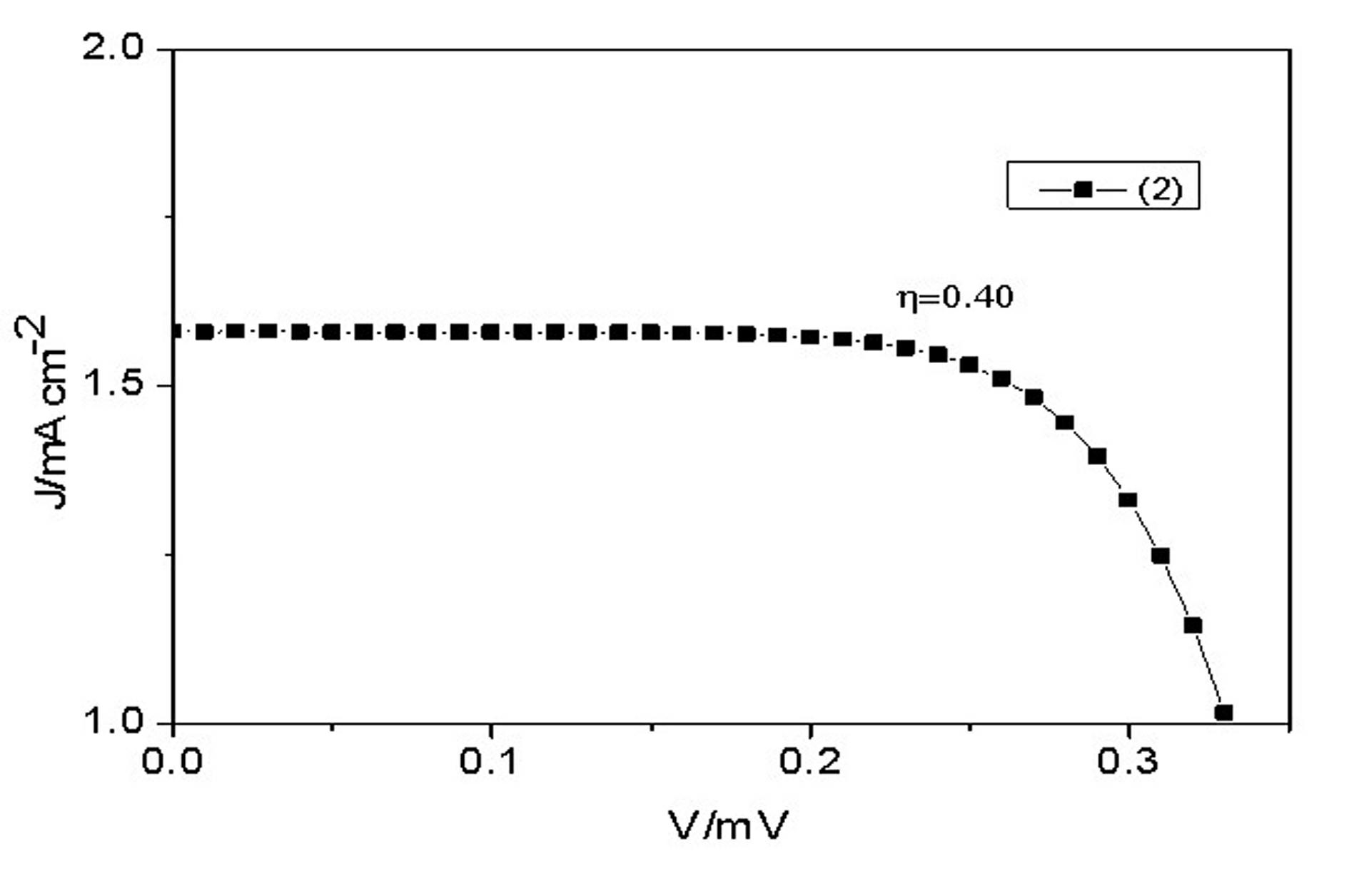
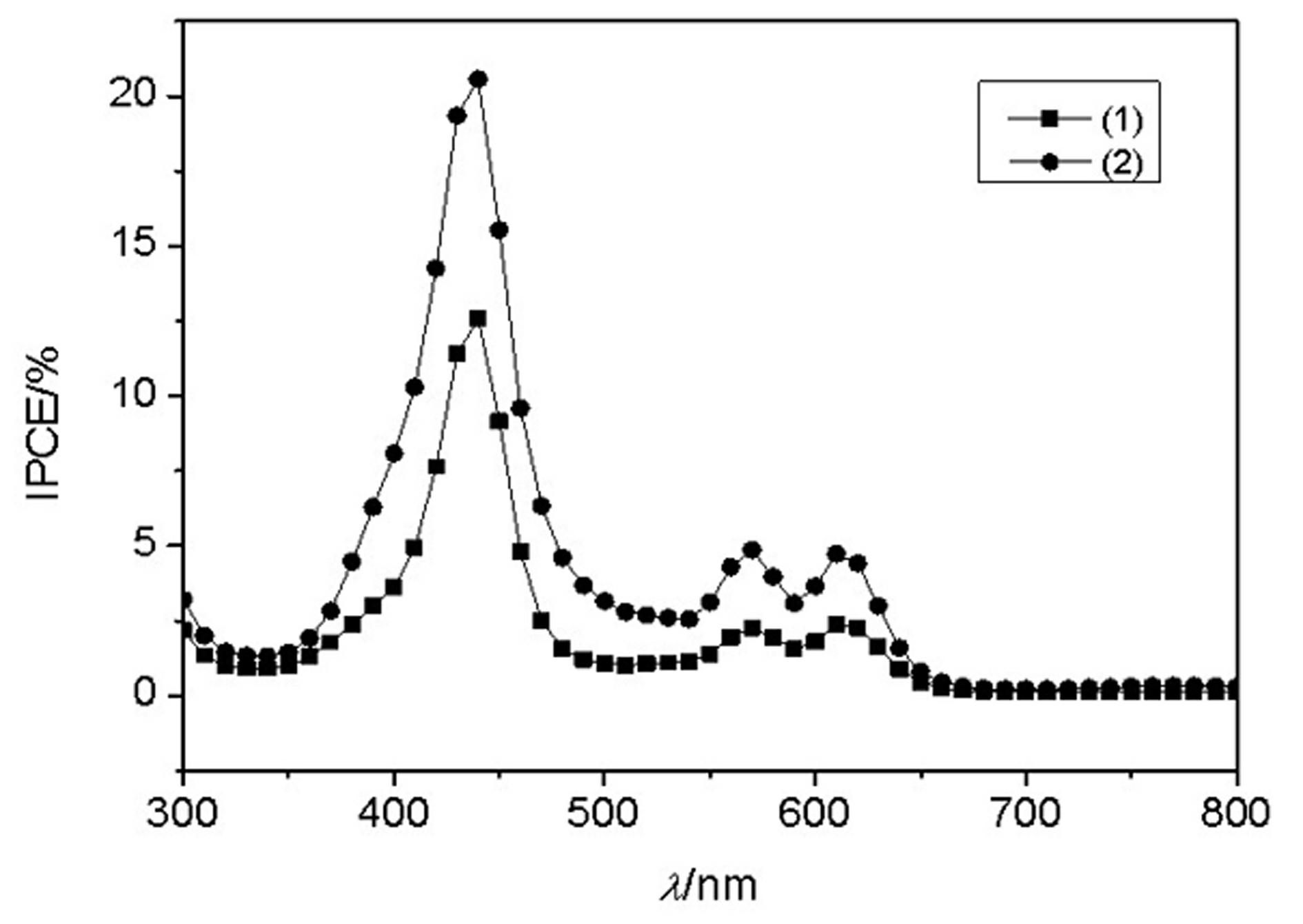
Conjugated Schiff base Zn (zinc) porphyrin as well as preparation and application thereof
A technology of Schiff base and conjugation, which is applied in the field of Schiff base conjugated Zn porphyrin compound and its synthesis, can solve the problems of limited resources and large-area use restrictions, and achieve low environmental pollution, low cost, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1, the synthesis of organic dye (1)
[0039] (1) Synthesis of 5,10,15,20-tetrakis(p-nitrophenyl)porphyrin
[0040] Add 4.0050g (26mmol) of p-nitrobenzaldehyde to 70ml of nitrobenzene, add 1.86ml (26mmol) of pyrrole, and when the temperature of the system reaches 180°C, inject 2.25ml (27mmol) of L-lactic acid and react for 2 hours. Cool to room temperature, add 30ml methanol and stir for 0.5h, place in the refrigerator, filter after 12h, wash the filter cake with methanol and acetone to get 5,10,15,20-tetrakis(p-nitrophenyl)porphyrin, the yield 19%.
[0041] (2) Synthesis of 5,10,15,20-tetrakis(p-aminophenyl)porphyrin
[0042] Dissolve 1.4556g (1.82mmol) of 5,10,15,20-tetrakis(p-nitrophenyl)porphyrin in 60ml of concentrated hydrochloric acid, add 5.8224g (25mmol) of stannous chloride in concentrated hydrochloric acid solution under the protection of argon , reacted in a water bath at 65°C for 1 h, changed to an ice bath for another 0.5 h, neutralized with a...
Embodiment 2
[0048] The synthesis of embodiment 2, organic dye (1)
[0049] Steps (1), (2), (3) are the same as in Example 1
[0050] (4) Synthesis of 5,10,15,20-tetrakis[(4-(2-pyridinealdehyde-benzimine)]-based zinc porphyrin (organic dye (1))
[0051] Dissolve 0.0677g (0.0884mmol) of 5,10,15,20-tetrakis(p-aminophenyl)zinc porphyrin and 0.7952mmol of 2-pyridinecarbaldehyde in 20ml of N,N-dimethylformamide under argon atmosphere Under protection, react at 80°C for 24h; after the reaction, cool to room temperature, add water and filter to obtain the crude product, wash the filter cake with water and methanol successively, and dry to obtain 5,10,15,20-tetrakis[(4-(2-pyridine Aldehyde-benzimine)] base zinc porphyrin, the productive rate is 49%.
[0052] The proton nuclear magnetic spectrum data are with embodiment 1.
Embodiment 3
[0053] Embodiment 3, the synthesis of organic dye (1)
[0054] Steps (1), (2), (3) are the same as in Example 1
[0055] (4) Synthesis of 5,10,15,20-tetrakis[(4-(2-pyridinealdehyde-benzimine)]-based zinc porphyrin (organic dye (1))
[0056] Dissolve 0.2257g (0.2946mmol) of 5,10,15,20-tetrakis(p-aminophenyl)zinc porphyrin and 2.6509mmol of 2-pyridinecarbaldehyde in 20ml of N,N-dimethylformamide under argon atmosphere Under protection, react at 80°C for 24h; after the reaction, cool to room temperature, add water and filter to obtain the crude product, wash the filter cake with water and methanol successively, and dry to obtain 5,10,15,20-tetrakis[(4-(2-pyridine Aldehyde-benzyl imine)] base zinc porphyrin, productive rate is 45%.Proton nuclear magnetic spectrum data are with embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com