Method for preparing capsicine monomer and dihydrocapsaicin std monomer
A technology of dihydrocapsaicin and capsaicin, applied in the field of biomedicine, can solve problems such as unfavorable industrialized production, cumbersome process steps, unfavorable large-scale production, etc., achieves solvent consumption, non-toxicity, environmental protection, high product yield and purity, and is suitable for large-scale production. The effect of mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Take homemade capsaicin, then add methanol and water to prepare a capsaicin raw material solution with a methanol content of 20% and a concentration of 1.04 mg / mL.
[0025] (2) Pack the pretreated ODS into the column by wet method, the column diameter is 1.1cm, the packing height is 60cm, and 10mL of sample is loaded at a flow rate of 0.5mL / min.
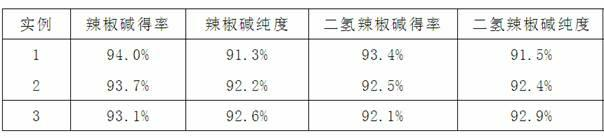
[0026] (3) After the raw material is completely absorbed, elute the column with 180mL of 75% methanol at a flow rate of 0.5ml / min, collect in sections, combine the capsaicin and dihydrocapsaicin components separately, and analyze the components by high performance liquid chromatography See Table 1 for the purity and yield (relative to the raw material solution).
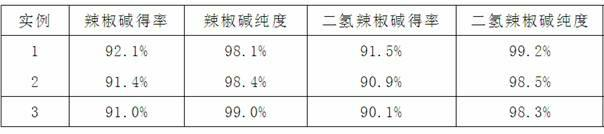
[0027] (4) After removing the solvent from the collected components, recrystallize with ethanol, and analyze the crystal purity and yield (relative to the raw material liquid) by high performance liquid chromatography, see Table 2.
Embodiment 2
[0029] (1) Take homemade capsaicin, then add methanol and water to prepare a capsaicin raw material solution with a methanol content of 30% and a concentration of 3.36 mg / mL.
[0030] (2) Pack the pretreated ODS into the column by wet method, the column diameter is 1.6cm, the packing height is 70cm, and 300mL of sample is loaded at a flow rate of 2.0mL / min.
[0031] (3) After the raw material is completely absorbed, elute the column with 400mL of 70% methanol at a flow rate of 1.5ml / min, collect in sections, combine the capsaicin and dihydrocapsaicin components separately, and analyze the components by high performance liquid chromatography The purity and yield are shown in Table 1.
[0032] (4) After removing the solvent from the collected components, recrystallize with ethanol, and analyze the crystal purity and yield by high performance liquid chromatography, see Table 2.
Embodiment 3
[0034] (1) Take homemade capsaicin, then add methanol and water to prepare a capsaicin raw material solution with a methanol content of 35% and a concentration of 4.71 mg / mL.
[0035] (2) Pack the pretreated ODS into the column by wet method, the column diameter is 4.0cm, the packing height is 70cm, and 2000mL of sample is loaded at a flow rate of 5.0mL / min.
[0036] (3) After the raw material is completely absorbed, elute the column with 2500mL of 70% methanol at a flow rate of 5.0ml / min, collect in sections, combine the capsaicin and dihydrocapsaicin components separately, and analyze the group by high performance liquid chromatography The purity and yield of the fractions are shown in Table 1.
[0037] (4) After removing the solvent from the collected components, recrystallize with ethanol, and analyze the crystal purity and yield by high performance liquid chromatography, see Table 2.
[0038] Table 1 Experimental data of components analyzed by high performance liquid chr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com