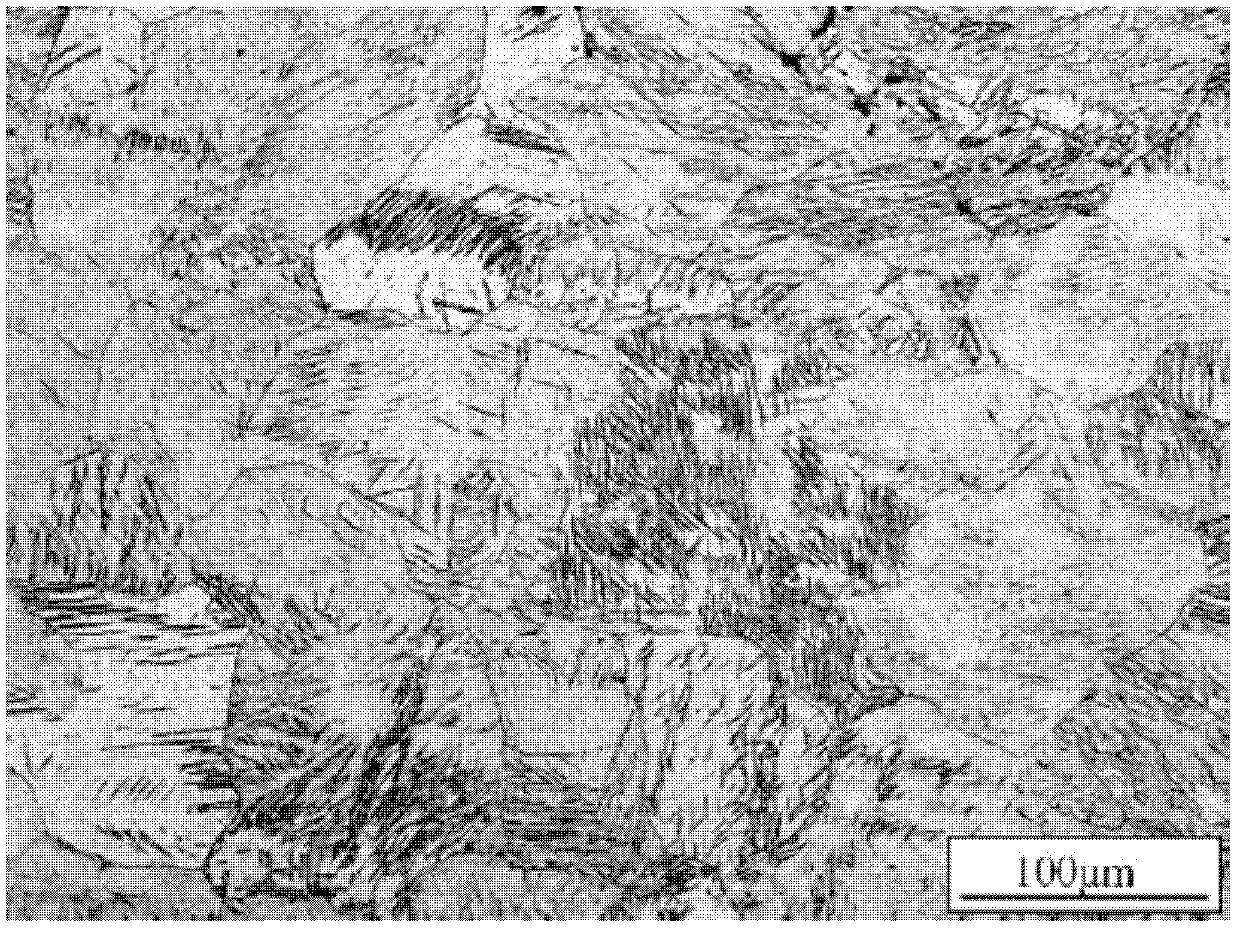
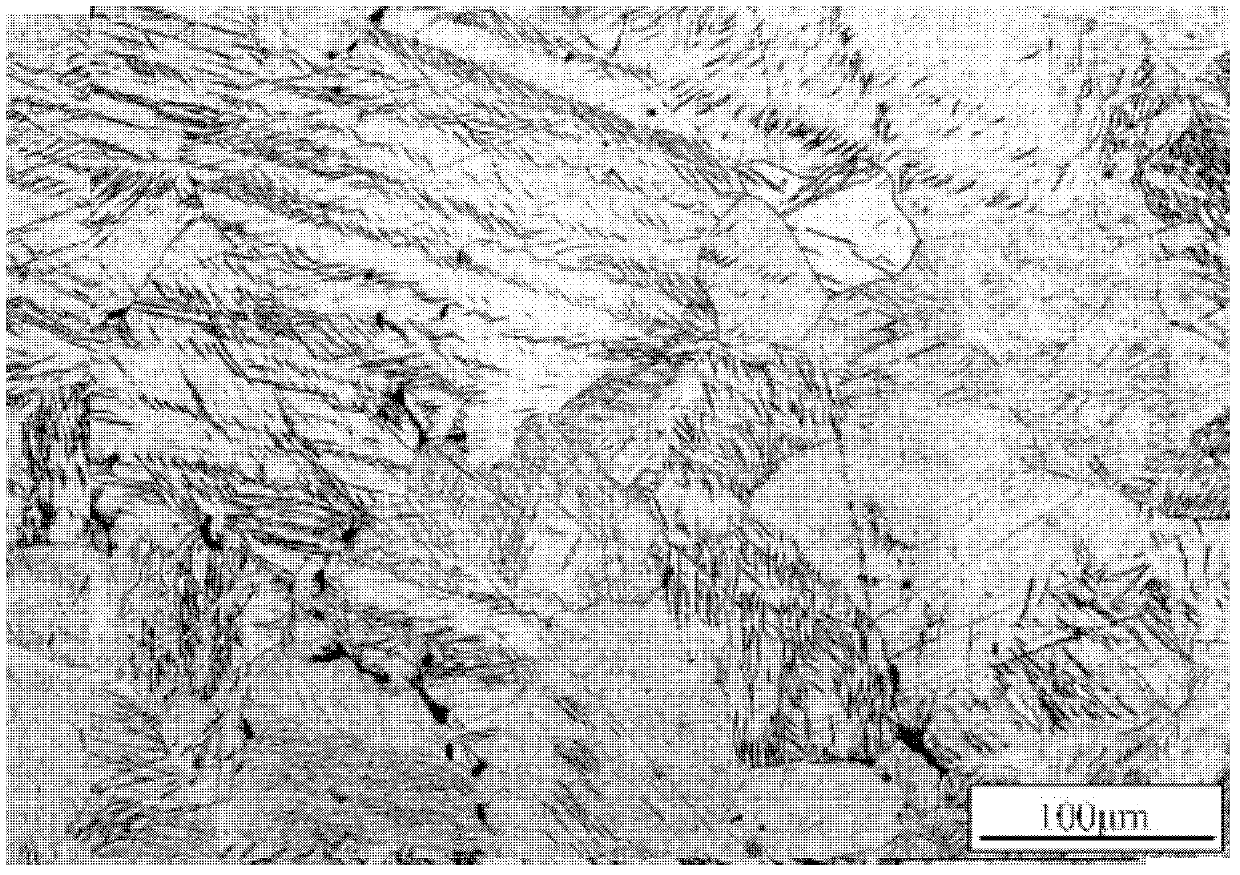
Biomedical Mg-Sn-Zn-Mn magnesium alloy and preparation method thereof
A biomedical and magnesium alloy technology, applied in the field of alloys, can solve the problems of long-term experimental observation and unclear biological effects, etc., and achieve good comprehensive mechanical properties, reduce stacking fault energy, and improve toughness.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Use pure magnesium ingots (purity not less than 99.96%), pure tin ingots (purity not less than 99.95%), pure zinc ingots (purity not less than 99.95%) and Mg-6.8% Mn master alloy, according to Mg-3Sn-1Zn-0.5 Mn alloy composition (nominal composition: Sn 3%, Zn 1%, Mn 0.5%, impurity elements Fe6 / CO 2 Under the protection of mixed gas, it is poured into the metal mold for gravity casting. Before the solution heat treatment, the above-mentioned Mg-3Sn-1Zn-0.5Mn alloy ingot is pre-deformed. The pre-deformation process is to put the above alloy ingot into the SF 6 / CO 2 Extrusion is performed after homogenization heat treatment (homogenization temperature is 300° C., holding time is 5 hours) in a heat treatment furnace mixed with protective gas, and the extrusion deformation is 10%. Finally, the pre-deformed alloy is subjected to solution heat treatment (solution treatment temperature is 450° C., holding time is 24 hours, furnace cooling). The tensile strength of the al...
Embodiment 2
[0038] Use pure magnesium ingots (purity not less than 99.96%), pure tin ingots (purity not less than 99.95%), pure zinc ingots (purity not less than 99.95%) and Mg-6.8% Mn master alloy, according to Mg-3Sn-1Zn-1Mn Alloy composition (nominal composition: Sn 3%, Zn 1%, Mn 1%, impurity elements Fe6 / CO 2 Under the protection of mixed gas, it is poured into the metal mold for gravity casting. Before the solution heat treatment, the above-mentioned Mg-3Sn-1Zn-1Mn alloy ingot is pre-deformed. The pre-deformation process is to put the above alloy ingot into the SF 6 / CO 2 Extrusion is performed after homogenization heat treatment (homogenization temperature is 300° C., holding time is 5 hours) in a heat treatment furnace mixed with protective gas, and the extrusion deformation is 5%. Finally, the pre-deformed alloy is subjected to solution heat treatment (solution treatment temperature is 410° C., holding time is 28 hours, air cooling). The tensile strength of the alloy at room ...
Embodiment 3
[0040] Using pure magnesium ingots (purity not less than 99.96%), pure tin ingots (purity not less than 99.95%), pure zinc ingots (purity not less than 99.95%) and Mg-6.8% Mn master alloy, according to Mg-3Sn-0.5Zn- 0.15Mn alloy composition (nominal composition: Sn 3%, Zn0.5%, Mn 0.15%, impurity elements Fe6 / CO 2 Under the protection of mixed gas, it is poured into the metal mold for gravity casting. Before the solution heat treatment, the above-mentioned Mg-3Sn-0.5Zn-0.15Mn alloy ingot is pre-deformed. The pre-deformation process is to put the above alloy ingot into the SF 6 / CO 2 Carry out homogenization heat treatment (homogenization temperature is 400 DEG C, holding time is 3 hours) in the heat treatment furnace of mixing shielding gas and then carry out rolling, rolling deformation amount is 10%. Finally, the pre-deformed alloy is subjected to solution heat treatment (solution treatment temperature is 450° C., holding time is 12 hours, air cooling). The tensile stren...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tensile strength | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com