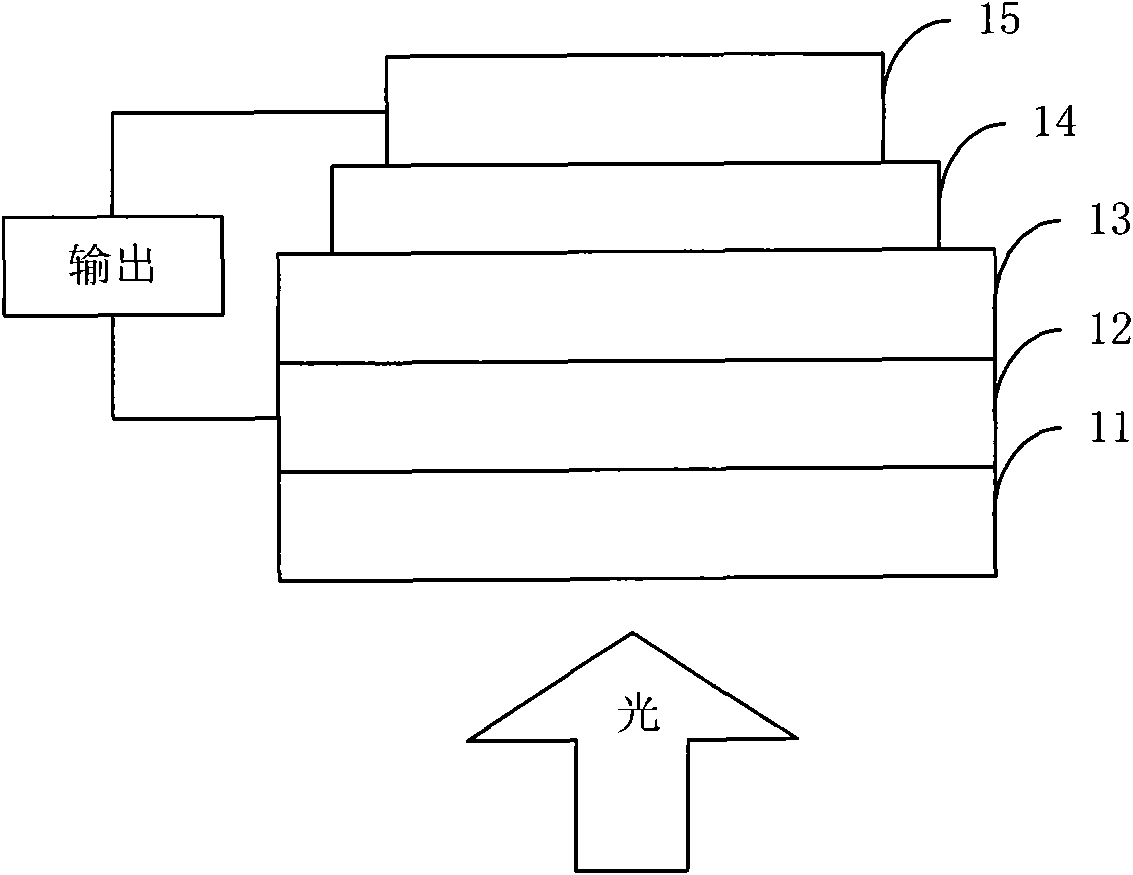
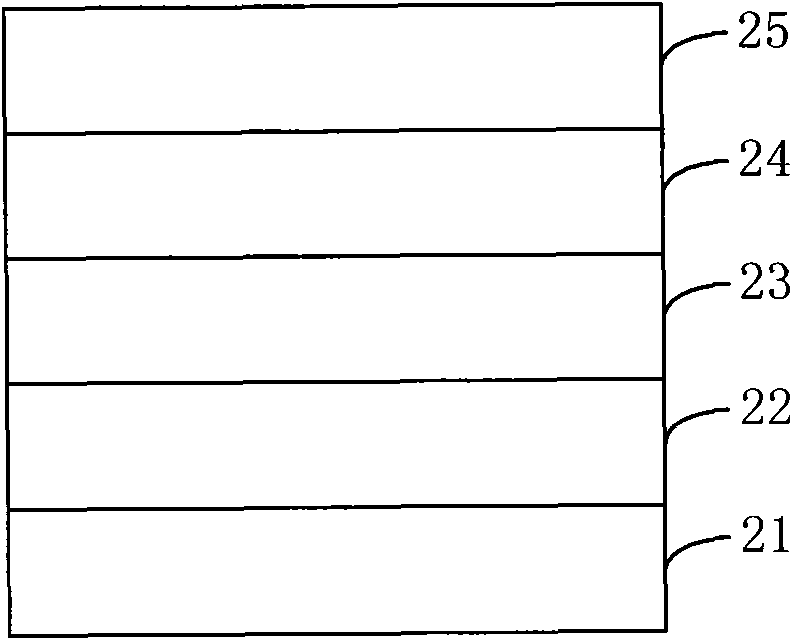
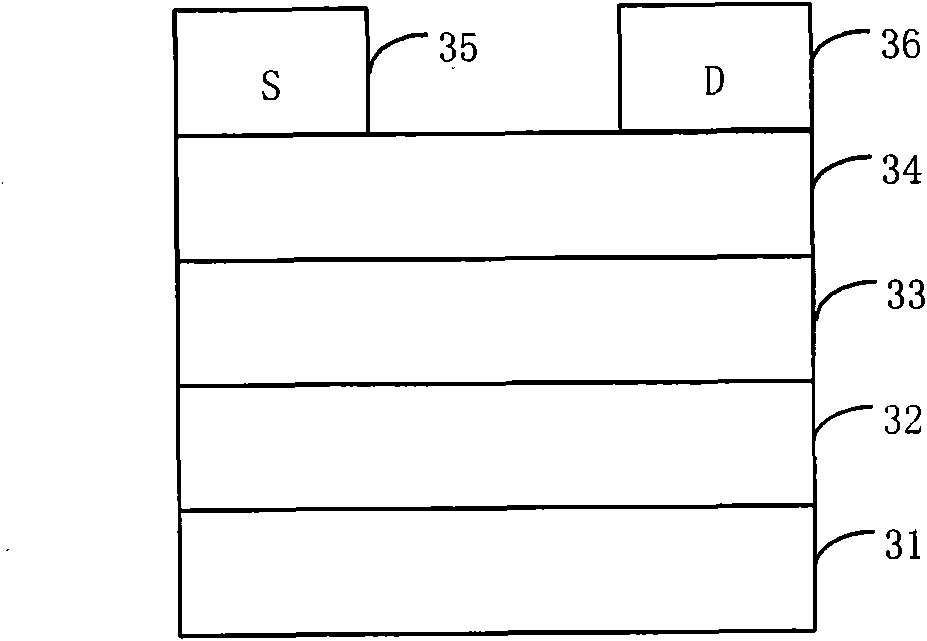
Organic semiconductor material containing thiophene pyrrole dione unit and preparation method and application thereof
A thiophene pyrrole diketo, organic semiconductor technology, applied in the field of organic semiconductor materials, can solve problems such as low photoelectric conversion efficiency, and achieve the effects of improving photoelectric conversion efficiency, being easy to control, and being beneficial to film forming processing.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The preparation method of the organic semiconductor material containing thienopyrrole diketone unit comprises the following steps:
[0033] Step S1: In an oxygen-free environment, the dithienothiophene compound (A 1 ) and n-butyllithium (n-BuLi) were added to the first solvent at a molar ratio of 1:2 to 1:4 at -100°C to -25°C to react for 0.5 to 5 hours, and then trimethyltin chloride ( Me 3 SnCl, the amount of which is 2 to 4 times the molar weight of the dithienothiophene compound), and the reaction was continued for 24 to 48 hours to obtain the product, namely (3,5-dialkyldithieno[3,2-b; 2 ',3'-d]thiophene-2,6-diyl)bis(trimethyltin)(B 1 ); wherein, the first solvent is at least one of tetrahydrofuran, ether, dichloromethane, chloroform or ethyl acetate, R 1 , R 2 same or different as C 1 -C 20 The alkyl group; Its reaction formula is as follows:
[0034]
[0035] Step S2: 5-alkylthiophene[3,4-c]-pyrrole-4,6-dione (A 2 ) and 1,3-dibromo-5-alkylthiophene [3,...
Embodiment 1
[0041] Embodiment 1. This embodiment discloses a copolymer with the following structure as an organic semiconductor material:
[0042]
[0043] In the above formula, n=20;
[0044] The preparation steps of above-mentioned copolymer are as follows:
[0045] One, the preparation of 4,4-dimethyl-2,6-bis(trimethyltin base)-4H-cyclopenta[2,1-b:3,4-b']dithiophene:
[0046]
[0047] At -100°C under nitrogen, add 20.00mL (2.00M) of n-butyllithium solution to 2.06g of 4,4-dimethyl-4H-cyclopenta[2,1-b:3,4- b'] In a reaction flask of dithiophene and 60 mL of tetrahydrofuran, after stirring for 1 hour, slowly add 7.74 g of trimethyltin chloride dropwise, return to room temperature, and continue stirring for 48 hours. After the reaction was completed, the reaction solution was poured into water, extracted with ether, dried over anhydrous magnesium sulfate, rotary evaporated, and recrystallized to obtain the product.
[0048] MALDI-TOF-MS (m / z): 531.9 (M + ).
[0049] Two, the pr...
Embodiment 2
[0061] Embodiment 2. This embodiment discloses a copolymer with the following structure as an organic semiconductor material:
[0062]
[0063] In the above formula, n=59;
[0064] The preparation steps of above-mentioned copolymer are as follows:
[0065] One, the preparation of 4,4-dioctyl-2,6-bis(trimethyltin base)-4H-cyclopenta[2,1-b:3,4-b']dithiophene:
[0066]
[0067] At -78°C under nitrogen, 20.00 mL (1.00 M) of n-butyllithium solution was added to 4.03 g of 4,4-dioctyl-4H-cyclopenta[2,1-b:3,4- b'] In a reaction flask of dithiophene and 70 mL of tetrahydrofuran, after stirring for 1 hour, slowly add 3.96 g of trimethyltin chloride dropwise, return to room temperature, and continue stirring for 32 hours. After the reaction was completed, the reaction solution was poured into water, extracted with ether, dried over anhydrous magnesium sulfate, rotary evaporated, and recrystallized to obtain the product.
[0068] MALDI-TOF-MS (m / z): 728.3 (M + ).
[0069] Two, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistance | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com