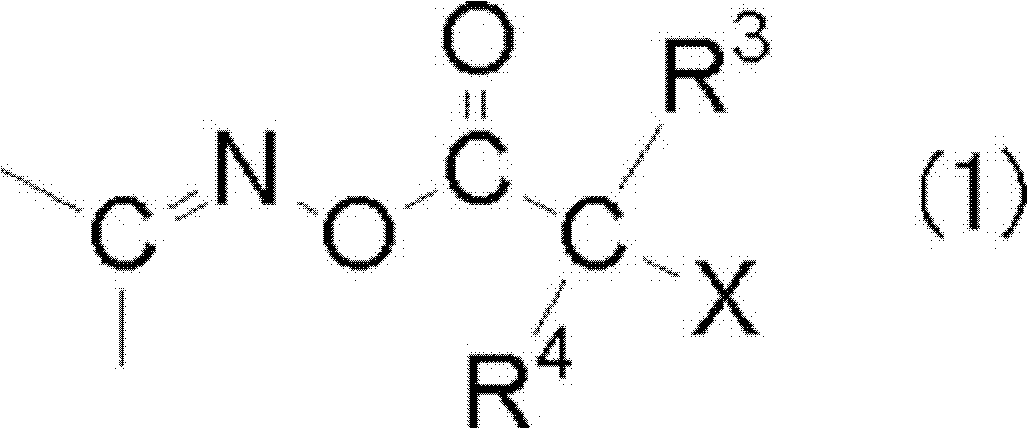Photopolymerizable composition, color filter, method for producing same, solid-state image pickup element, liquid crystal display device, lithographic printing original plate, and novel compound
一种光聚合性、组合物的技术,应用在光聚合性组合物,新型化合物领域,能够解决灵敏度不足、曝光时间变长、成品率降低等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0415] Hereinafter, although an Example demonstrates this invention more concretely, this invention is not limited to the following Example unless the summary is exceeded. In addition, unless otherwise indicated, "part" means a mass basis, and "%" means "mass %".
[0416] First, details of the specific oxime compounds (Specific Compound 1 to Specific Compound 11) used in Examples and comparative compounds (Comparative Compound 1 to Comparative Compound 4) used in Comparative Examples are given.
[0417] The synthesis method of Specific Compound 1 to Specific Compound 11 is shown below.
[0418]
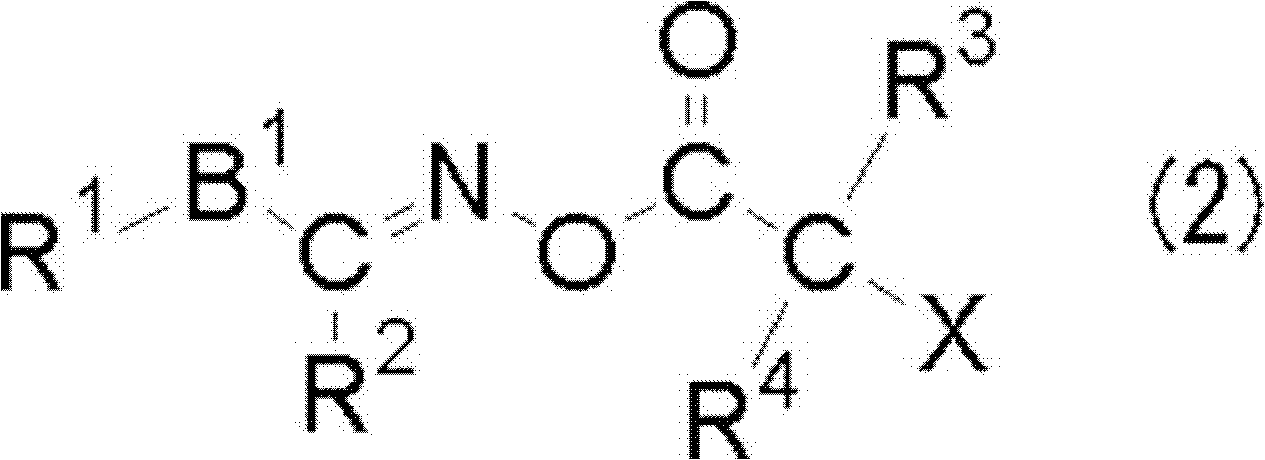
[0419]
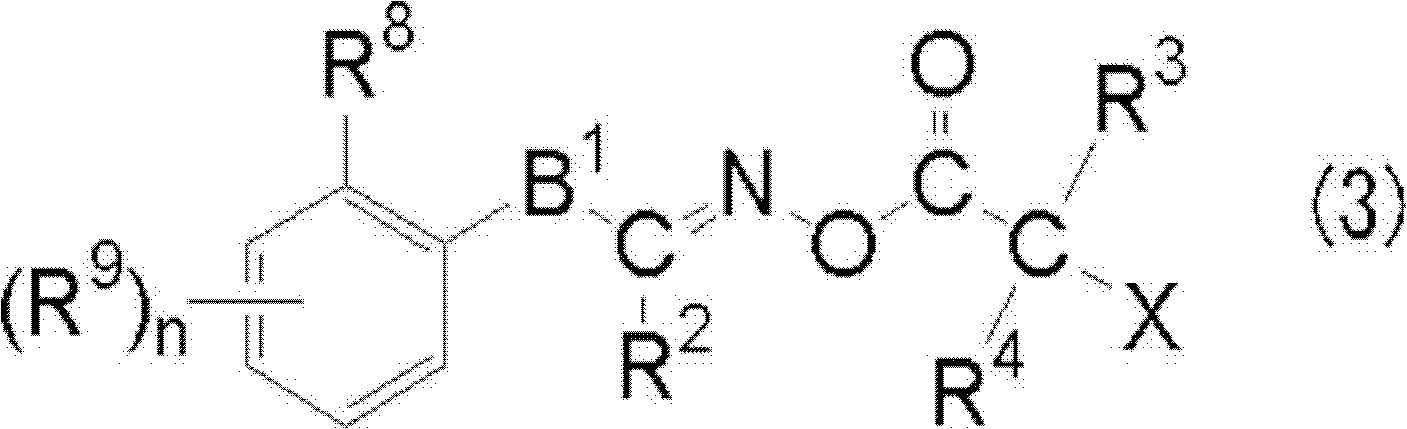
[0420]
[0421] (Synthesis of α Heterocompounds)
[0422] ·Synthesis of α-morpholino isobutyrate hydrochloride
[0423] Dilute ethyl 2-bromopropionate (0.125 mol) in 75 mL of toluene, add formalin (0.3 mol), and stir at room temperature for 10 hours. After removing the white solid by filtration, the filtrate was distilled to obtain ethyl 2-morpholinopropionate (0....
Synthetic example 1
[0435] [Synthesis Example 1] Synthesis of Specific Compound 1 as Specific Oxime Compound
[0436] 1-[6-(2-methylbenzoyl)-9-ethyl-9H-carbazol-3-yl]ethanone oxime (12mmol) and α-morpholino isobutyrate hydrochloride (12mmol ) was suspended in 20 mL of dichloromethane. After cooling this to 0°C, 4-dimethylaminopyridine (24 mmol) and dicyclohexylcarbodiimide (13 mmol) were added thereto, followed by stirring at 0°C for 1 hour. After extraction with chloroform, it was washed with a saturated aqueous sodium chloride solution, the organic layer was dried over magnesium sulfate, and the solvent was distilled off. The obtained residue was purified by silica gel column chromatography (hexane / ethyl acetate=4 / 1) to obtain 7.6 mmol of the target specific compound 1 .
[0437] The structure of the obtained specific compound 1 was identified by NMR.
[0438] ( 1 H-NMR 300MHz deuterated chloroform): 1.47(s, 6H,), 1.49(t, 3H, J=7.2Hz), 2.35(s, 3H), 2.52(s, 3H), 2.74(t, 4H, J =4.5Hz), 3.75(...
Synthetic example 2
[0440] [Synthesis Example 2] Synthesis of Specific Compound 2 as Specific Oxime Compound
[0441] In Synthesis Example 1, it was synthesized by the same method as Specific Compound 1, except that α-diethylaminoisobutyrate hydrochloride was used instead of α-pyrrolidine isobutyrate hydrochloride.
[0442] The structure of the obtained specific compound 2 was identified by NMR.
[0443] ( 1 H-NMR 300MHz deuterated chloroform): 1.45(s, 6H,), 1.49(t, 3H, J=7.2Hz), 1.55(t, 6H, J=7.2Hz), 2.35(s, 3H), 2.54( s, 3H), 2.90(m, 4H), 4.42(q, 2H, J=7.2Hz), 7.26~7.47(m, 6H), 7.98(d, 1H, J=8.4Hz), 8.11(d, 1H , J=8.4Hz), 8.45(s, 1H), 8.52(s, 1H)
[0444] The molar absorptivity at 365 nm of Specific Compound 2 was measured in the same manner as above, and it was 1600 in ethyl acetate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| coating mass | aaaaa | aaaaa |
| roughness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com