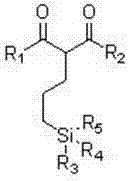
Organic silicon coupling agent containing beta-dicarbonyl and preparation method thereof
A silicone coupling agent, dicarbonyl technology, applied in the field of preparation of new high temperature resistant silicone coupling agent, the silicone coupling agent can solve the problems of low-grade processing, limited use range, long production process, etc. , to achieve the effect of unique performance, low cost and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
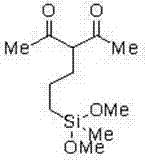
[0042] Embodiment 1, the preparation of diethyl 2-(3-dimethoxy (methyl) silicon) propane) ethyl ester
[0043](1) Preparation of diethyl allylmalonate
[0044] Add anhydrous potassium carbonate (8.58g, 61mmol), diethyl malonate (7.68g, 60mmol), allyl chloride (4.64g, 65mmol) into a 150 mL round-bottomed flask in turn, and then add polar organic Solvent acetonitrile 70mL, control the temperature at 80°C, reflux and stir for 24 hours, take it out and cool to room temperature, add water to remove excess potassium carbonate, extract with ethyl acetate, wash the organic phase with water 3 times, dry over anhydrous sodium sulfate, and vacuum rotary evaporation The solvent was spin-dried to obtain a yellow transparent liquid as the product (directly used in the next reaction without further purification), with a yield of 95%.
[0045] The resulting product allyl malonate diethyl ester structure is:
[0046]
[0047] The NMR data are as follows:
[0048] 1 H NMR (400 MHz, CDCl...
Embodiment 2
[0056] The preparation of embodiment 2,2-(3-dimethoxy (methyl) silicon) propane) acetylacetone
[0057] (1) Preparation of allyl acetylacetone
[0058] Add anhydrous potassium carbonate (8.58g, 61mmol), acetylacetone (6g, 60mmol), allyl chloride (4.64g, 65mmol) into a 150 mL round bottom flask in turn, and then add 70 mL of acetonitrile, a polar organic solvent, into the flask In the medium, control the temperature at 80°C, reflux and stir for 24 hours, take it out and cool to room temperature, add water to remove excess potassium carbonate, extract with ethyl acetate, wash the organic phase with water 3 times, dry over anhydrous sodium sulfate, and vacuum rotary evaporator The solvent was spin-dried to obtain a yellow transparent liquid which is the product allyl acetylacetone (directly used in the next step reaction without further purification), with a yield of 92%. Its structure is:
[0059]
[0060] The NMR data are as follows:
[0061] 1 H NMR (400 MHz, CDCl 3 ): ...
Embodiment 4
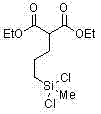
[0076] Embodiment 4, the preparation of diethyl 2-(3-dichloro(methyl)silyl)propane) ethyl ester
[0077] (1) Preparation of diethyl allylmalonate: same as Example 1.
[0078] (2) Preparation of diethyl 2-(3-dichloro(methyl)silyl)propane)ethyl ester
[0079] In a 50 mL round bottom flask, the platinum metal catalyst platinum-vinylsilane complex Pt 2 (Me 2 (CH=CH 2 ) 2 Si 2 O) 3 (4.74mg) was diluted in 30mL of desulfurized toluene, then 50mmol of dichloromethylsilane and 10mmol of allyl diethylmalonate were mixed and added dropwise to the flask, the temperature was controlled at 40°C, and stirred for 3~ After 6 hours, distill and collect fractions to obtain the product diethyl 2-(3-dichloro(methyl)silyl)propane)ethyl ester. Yield 56%. Its structure is:
[0080]
[0081] The NMR data are as follows:
[0082] 1 H NMR (400 MHz, CDCl 3): d = 4.12-4.11 (m, 4H), 3.15-3.13 (m, 1H), 2.03-2.02 (m, 2H), 1.38-1.36 (m, 2H), 1.35-1.34 (m, 2H), 1.30 -1.28 (m, 6H), 0.79-0.7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com