Carbonyl reductase and gene thereof as well as application of carbonyl reductase in asymmetrical reductive carbonyl compound
A technology of carbonyl reductase and reductase, which is applied in the field of bioengineering technology and can solve problems such as poor substrate tolerance, low enzyme catalytic activity, and insufficient product concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Cloning of embodiment 1 reductase gene
[0064] According to the gene sequence (Genebank accession number: CAG61069.1) recorded in Genbank and predicted as Candida glabrata reductase, the PCR primers were designed as follows:
[0065] Upstream primer: CGC GGATCC ATGGTTAAGCAAGAATTCTTT;
[0066] Downstream primer: GTG AAGCTT TTAGGCCTTCTGGGACTCTGA.
[0067] Wherein, the underlined part of the upstream primer is the BamHI restriction site, and the underlined part of the downstream primer is the HindIII restriction site.
[0068] The genomic DNA of Candida glabrata CGMCC 2.234 was used as a template for PCR amplification. PCR system: 10 μl of 2×Taq PCR MasterMix, 1 μl of upstream primer and downstream primer (0.3 μmol / L), 1 μl of DNA template (0.1 μg) and ddH 2 O 7 μl. PCR amplification steps are: (1) Pre-denaturation at 95°C for 3 minutes; (2) Denaturation at 94°C for 1 minute; (3) Annealing at 55°C for 30 seconds; (4) Extension at 72°C for 1.5 minutes; steps (2) to ...
Embodiment 2
[0069] Embodiment 2 Preparation of recombinant expression vector (plasmid) and recombinant expression transformant
[0070] The reductase gene DNA fragment obtained in Example 1 was double-digested with restriction endonucleases BamHI and HindIII at 37°C for 12 hours, purified by agarose gel electrophoresis, and the target fragment was recovered using an agarose gel DNA recovery kit. Under the action of T4 DNA ligase, the target fragment was ligated with the plasmid pET28a digested with BamHI and HindIII at 4°C overnight to obtain the recombinant expression plasmid pET-CgKR2.
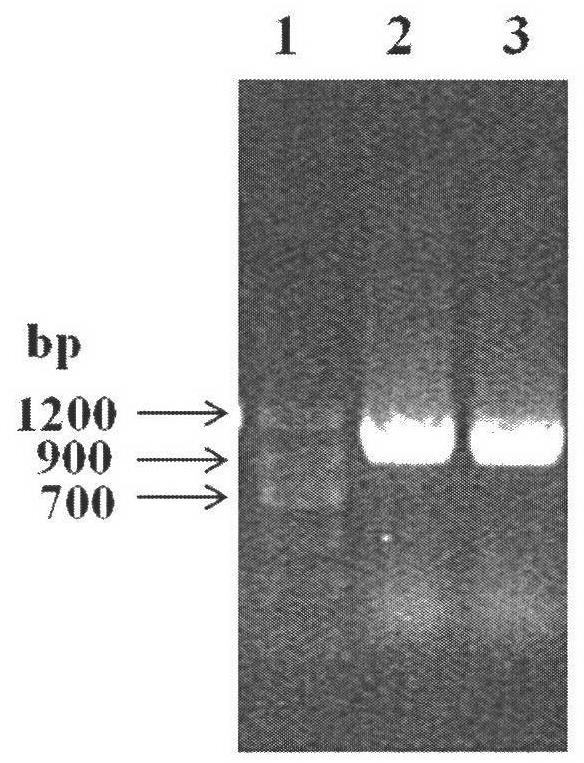
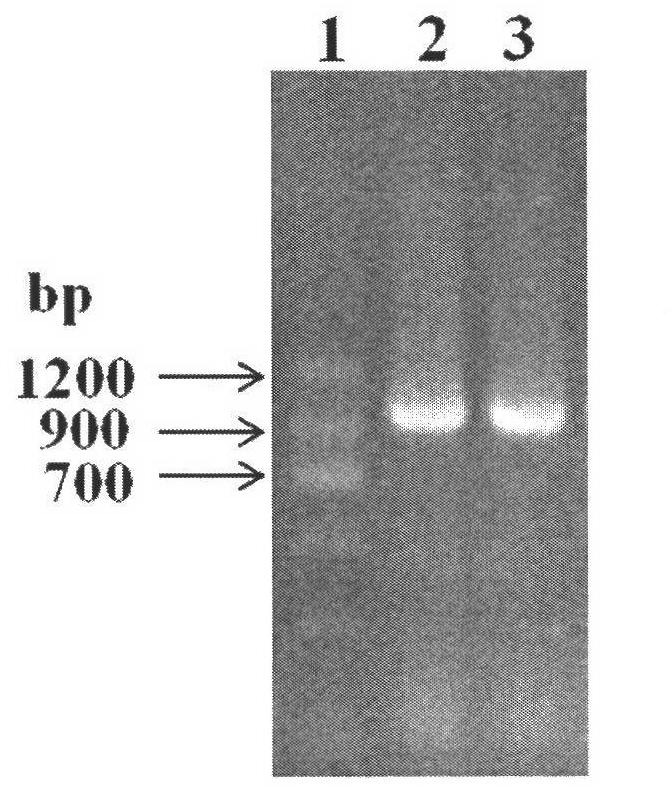
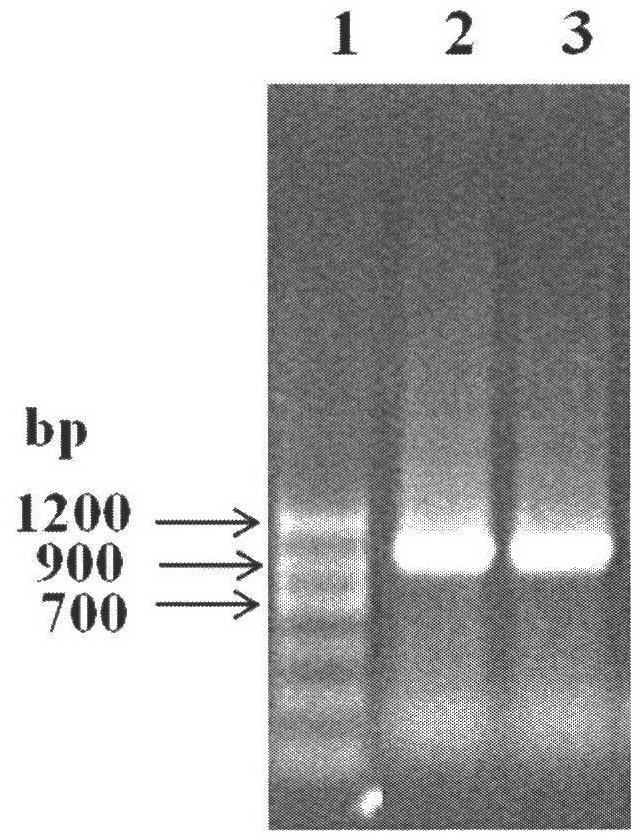
[0071] Transform the above-mentioned recombinant expression plasmid into Escherichia coli (E.coli) DH5α competent cells, screen the positive recombinants on the resistance plate containing kanamycin, pick a single clone, and the colony PCR verification is positive clone( figure 2 ). Cultivate the recombinant bacteria, extract the plasmid after the plasmid is amplified, retransform into Escherichia co...
Embodiment 3
[0072] Embodiment 3 expression of recombinant reductase
[0073] The recombinant Escherichia coli obtained in Example 2 was inoculated into LB medium containing ampicillin (peptone 10g / L, yeast extract 5g / L, NaCl 10g / L, pH 7.0), cultured with shaking at 37°C overnight, and 1% The inoculation amount of (v / v) is inserted in the 500ml Erlenmeyer flask that 100ml LB culture medium is housed, put 37 ℃, 180rpm shaker shaking culture, when the OD of culture solution 600 When it reached 0.6, IPTG with a final concentration of 0.5 mmol / L was added as an inducer, and after induction at 25°C for 12 hours, the culture medium was centrifuged to collect cells and washed twice with saline to obtain resting cells. The resulting resting cells were suspended in a pH 7.0 buffer, ultrasonically disrupted in an ice bath, and the supernatant was collected by centrifugation, which was the crude enzyme solution of the recombinant reductase. The crude enzyme solution was analyzed by polyacrylamide ge...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com