Polycarboxylic acid hyper-dispersant for dispersing lithium iron phosphate precursor
A polycarboxylate-based, lithium iron phosphate technology, applied in electrical components, circuits, battery electrodes, etc., can solve problems such as a large number of bromide ions, increasing bromide ion content, and affecting sintering quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
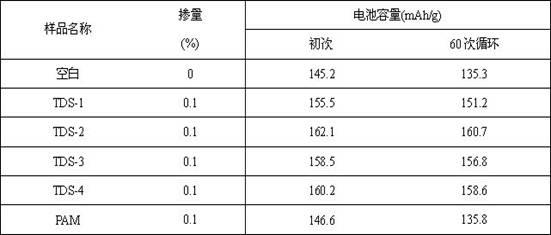
[0030] Add 300 parts of isopentenol polyoxyethylene ether and 140 parts of water in the reaction kettle, and keep stirring to make them all dissolved in water; then add 1.6 parts of hydrogen peroxide and stir for half an hour. Take 0.3 parts of terpinene and 1.2 parts of isopropanol, dissolve them in 140 parts of water, as drop A; take 48 parts of methacrylic acid and dissolve them in 120 parts of water, as drop B; within 3 to 4 hours, simultaneously Add dropwise to the reaction kettle evenly, and ensure that the dropwise addition of A is half an hour later than the dropwise addition of B. During this time, the temperature will rise slightly, and it can be controlled not to exceed 40 °C. After adding, continue stirring for 1 hour, add 24 parts of diethanolamine, and continue stirring for 30 minutes to obtain the product (TDS-1).
Embodiment 2
[0032] Add 310 parts of isopentenol polyoxyethylene ether and 140 parts of water in the reaction kettle, and keep stirring to make them all dissolved in water; then add 1.5 parts of hydrogen peroxide and stir for half an hour. Take 0.7 part of vinyl acetate and 1.0 part of ascorbic acid, dissolve it in 138 parts of water, as drop A; take 38 parts of acrylic acid and dissolve it in 125 parts of water, as drop B; in 3 to 4 hours, at the same time, uniform dropwise addition reaction In the kettle, ensure that the dripping of A is half an hour later than the dripping of B. During this time, the temperature will rise slightly, and it can be controlled not to exceed 40 °C. After adding, continue stirring for 1 hour, add 20 parts of ethanolamine, and continue stirring for 30 minutes to obtain the product (TDS-2).
Embodiment 3
[0034] Add 380 parts of isopentenol polyoxyethylene ether and 115 parts of water in the reaction kettle, and keep stirring to make them all dissolved in water; then add 1.6 parts of tert-butyl hydrogen peroxide and stir for half an hour. Take 0.6 parts of isopropanol and 1.2 parts of hypophosphorous acid, dissolve in 130 parts of water, as drop A; take 40 parts of acrylic acid and dissolve in 80 parts of water, as drop B; within 3~4 hours, add dropwise evenly at the same time In the reaction kettle, ensure that the dripping of A is half an hour later than the dripping of B. During this time, the temperature will rise slightly, and it can be controlled not to exceed 40 °C. After adding, continue stirring for 1 hour, add 22 parts of triethanolamine, and continue stirring for 30 minutes to obtain the product (TDS-3).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com