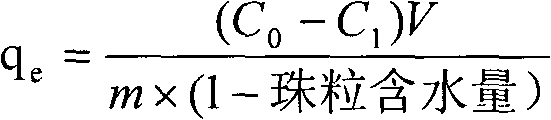Method for preparing di-dithiocarbamate modified chitosan beads
A technology of bis-dithiocarbamate and dithiocarbamate, which is applied in the field of preparation of bis-dithiocarbamate-modified chitosan beads, can solve the problem of easy dissolution and adsorption easily affected by pH, limitations Chitosan application and other issues, to achieve the effect of easy adsorption and recovery, good effect and good dispersibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
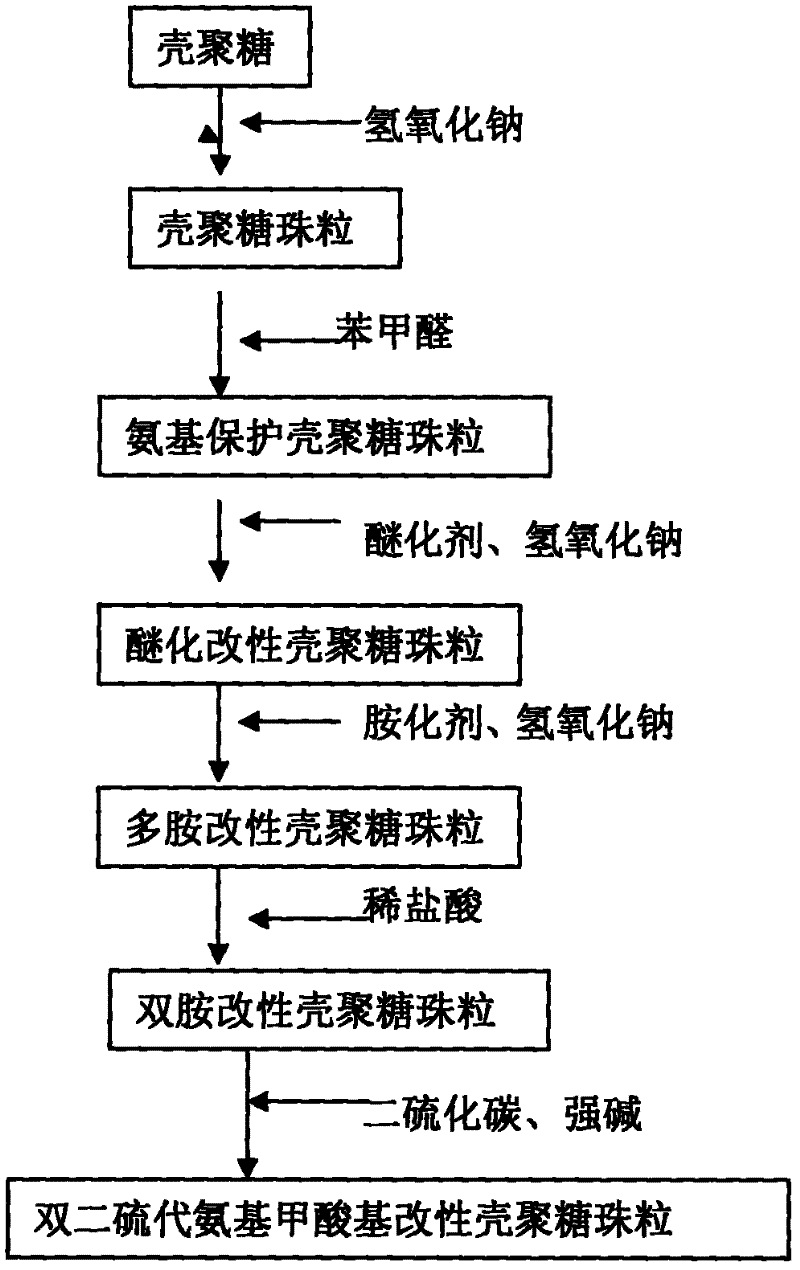
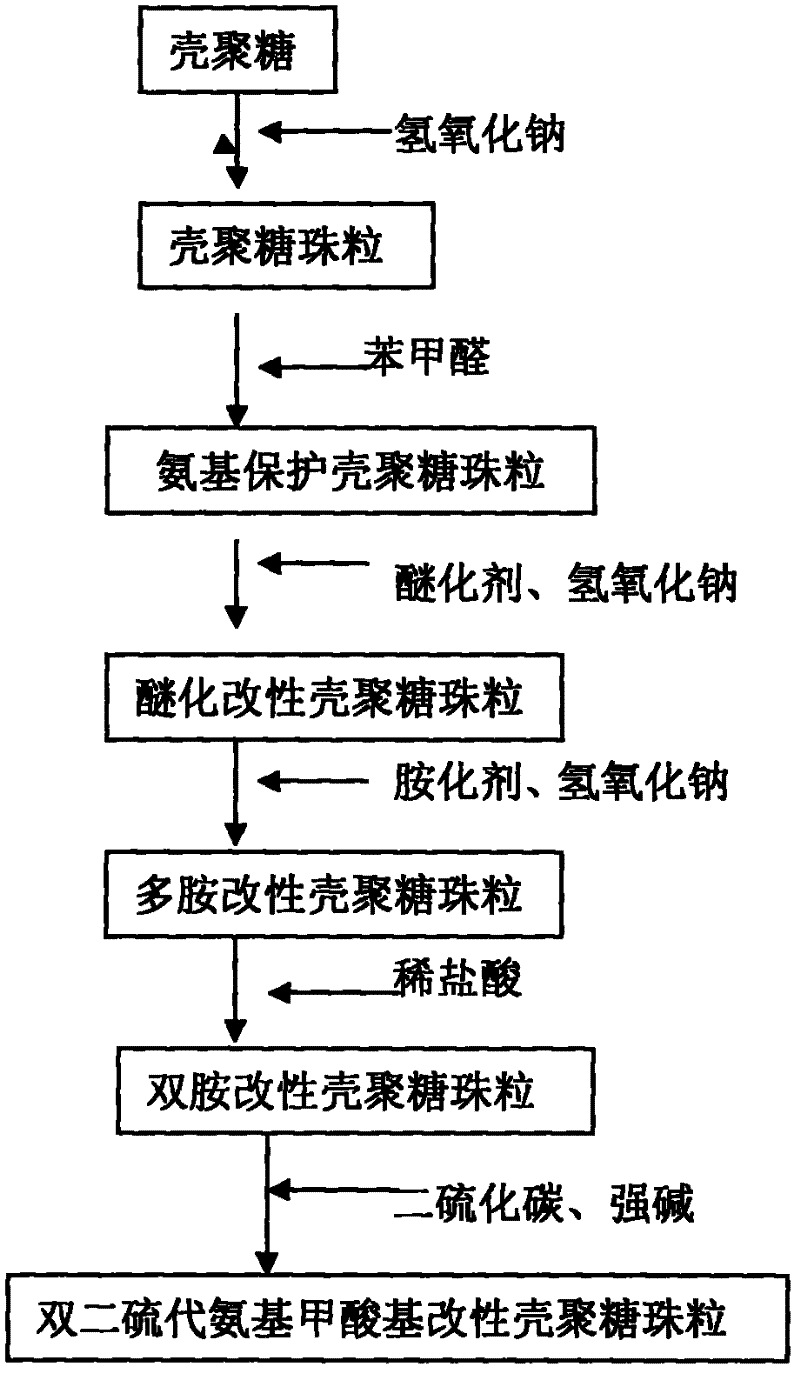
[0023] Step 1: Dissolve 5g of commercially available chitosan in 200mL of 2% dilute acetic acid solution. After dissolution, let it stand for 16 hours to remove bubbles. Pour the dissolved chitosan solution into an acid burette. Into the 2mol / L, 200mL sodium hydroxide solution drop by drop under slow stirring at room temperature. After dropping and standing for 16-24 hours, the chitosan beads are solidified to form chitosan beads, and the chitosan beads are fully washed with distilled water until the pH of the washed distilled water does not change, and chitosan beads are prepared.
[0024] In the second step, pour the chitosan beads washed with distilled water into a three-necked flask, add 200 mL of methanol and 20 g of benzaldehyde solution, and stir at room temperature for 16-24 hours. After the reaction, it is filtered, washed with ethanol and methanol to remove unreacted benzaldehyde, and finally washed with distilled water until the washing water is neutral to obtain amino...
Embodiment 2
[0031] The first step is the same as in Example 1.
[0032] In the second step, the chitosan beads washed with distilled water were poured into a three-necked flask, and 26 g of benzaldehyde was slowly added dropwise to the solution under constant stirring, and 250 mL of methanol was added. At room temperature, stirring was continued for 12 hours and then washed repeatedly with ethanol to remove unreacted benzaldehyde and the washing method was the same as that in Example 1. Put the obtained amino-protected chitosan beads into a beaker filled with distilled water and set aside.
[0033] Step 3. Add the amino-protected chitosan beads prepared in step 2 to 300mL 2molL -1 Add 40 mL of epichlorohydrin slowly to the sodium hydroxide solution under constant stirring. After reacting for 24 hours at room temperature, it was washed with hydrochloric acid to neutrality, and washed repeatedly with distilled water and ethanol to remove unreacted epichlorohydrin to obtain etherified modified ch...
Embodiment 3
[0039] At room temperature, weigh 3 parts of bisdithiocarbamate-modified chitosan beads, each part of bisdithiocarbamate-modified chitosan beads is 0.4g, and add them to 25mL of copper and nickel. In a heavy metal solution with a content of 100mg / L of zinc and zinc, the reaction was carried out on a magnetic stirrer for 12 hours under the condition of pH 4.0. After detection and calculation, the bisdithiocarbamate-based modified chitosan beads The saturated adsorption capacity of copper reaches 130 mg / g, and the saturated adsorption capacity of nickel and zinc exceeds 80 mg / g. However, using chitosan to replace the didithiocarbamate-based modified chitosan beads of the present invention will not The saturated adsorption capacity of copper ions is less than 100mg / g, and the saturated adsorption capacity of nickel and zinc are both less than 50mg / g. It shows that the didithiocarbamate-based modified chitosan beads obtained by the present invention have strong adsorption capacity ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Saturated adsorption capacity | aaaaa | aaaaa |
| Saturated adsorption capacity | aaaaa | aaaaa |
| Saturated adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com