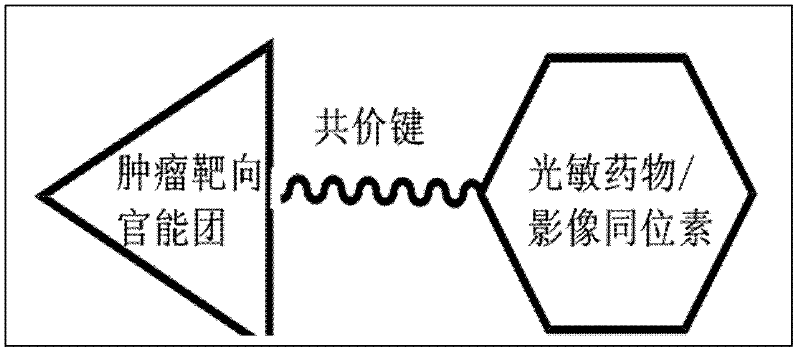
Medicines for targeted diagnosis and photosensitive therapy for cancer and application for same
A technology of photosensitizing drugs and photosensitizing compounds, which is used in cancer targeted diagnosis and photosensitizing therapeutic drugs and its application fields, and can solve the problem that photosensitizers can only reach the intercellular space, sample preparation of macromolecular carrier systems, chemical and biological stability and sample preparation. Storage issues, limited tumor uptake, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1, PZ-001-Ce6 and PZ-001-Ce6 / 64 Preparation of Cu
[0055] Photosensitizer-carrier copolymer PZ-001-Ce6 and PZ-001-Ce6 / 64 The chemical synthesis route of Cu is shown below (Scheme 1):
[0056]
[0057] Chemical synthesis route (Scheme 1)
[0058] N-a-(tert-butoxycarbonyl)-lysine was purchased from Sigma-Aldrich Chemicals.
[0059] N,N'-dicyclohexylcarbodiimide (DCC), N-hydroxysuccinimide, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and sodium tetraborate buffer solution were purchased from Arcos Chemicals.
[0060] Chlorin e6 (Ce6) was a gift from Medkoo Biosciences.
[0061] MHI-148 was synthesized according to literature procedures.
[0062] [64Cu]copper chloride was purchased from Washington University in St. Louis.
[0063] (1) Synthesis of MHI-148-lysine 2:
[0064] MHI-148 (200 mg, 0.28 mmol) and N-hydroxysuccinimide (32 mg, 0.28 mmol) were dissolved in 140 mL of dichloromethane, then 57.3 mg (0.28 mmol) of DCC were added. The mixt...
Embodiment 2
[0068] Example 2: PZ-001-Ce6 / 64 Synthesis of Cu 4b (or 4a)
[0069] Continuously add 100 µg of PZ-001-Ce63 in 1.5 ml eppendorf vials, 200 UL to achieve 0.1 N ammonium acetate buffer (pH 5.5) and 2 mCi [ 64 Cu]CuCl 2 . The reaction mixture was shaken at room temperature for 15 minutes. Purified by high performance liquid chromatography to obtain 1.8 mCi PZ-001-CE6 / 64 Cu4b (radiolabeling 90 to 96%). By a similar method, the corresponding non-radioactive compound 4a can be obtained. The mass spectrum (MALDI-TOF) of 4a is m / z 1451 (M+H).
Embodiment 3
[0070] Embodiment 3: Determination of singlet oxygen quantum yield (Δφs)
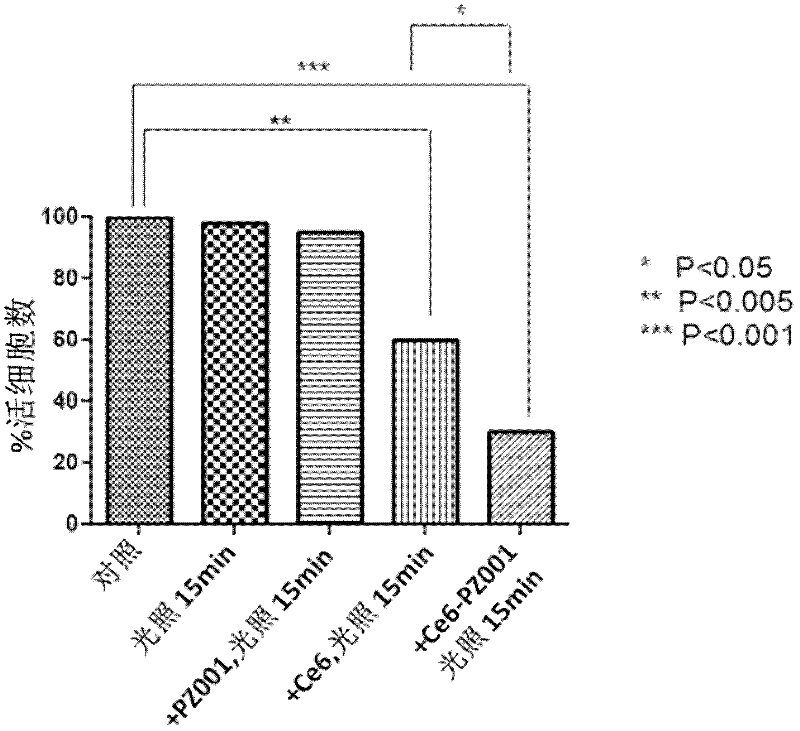
[0071] The determination of singlet oxygen quantum yield (Δφs) was performed according to the method of reference [13]. Briefly, 9,10-diphenyl was added to a tetrahydrofuran solution of PZ-001-Ce6, and oxygen was bubbled for 5-10 minutes. The prepared solution was then illuminated with a 655 nm laser beam (20 mW) (5 minutes). Measure the absorbance of the solution at 375 nm before and after illumination, and record the readings. The quantum yield of singlet oxygen can be determined by the difference in absorbance value, the formula is as follows: Δφs=ΔAS / ΔAR×ΔφR×100%. The results showed that the singlet oxygen quantum yield (PSC) of PZ-001-CE6 was 0.26%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com