Classical swine fever virus recombinant E2 protein and IgM (immune globulin M) antibody ELISA (enzyme-linked immunosorbent assay) test kit thereof
A swine fever virus and protein technology, applied in the field of molecular biology, to achieve the effect of good repeatability and good antigenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
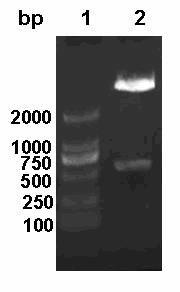
[0029] Example 1 PCR Amplification of E2 Gene Fragment and Construction of Prokaryotic Expression Plasmid
[0030] 1.1 Primer design
[0031] Primers were designed according to the CSFV gene sequence, and the upstream and downstream primers were respectively introduced Eco RI and Hind Site III, the sequence is as follows:
[0032] F: CCGGAATTCCGGCTGTGCCCGTTTGATACGAGT C (SEQ ID NO. 3);
[0033] R: CAAGCTTGTCTTTAGGTCTGCATGGCATAGG (SEQ ID NO. 4)
[0034] The above primers were synthesized by Nanjing Sipujin Biotechnology Co., Ltd.
[0035] 1.2 PCR amplification of the target fragment
[0036] Take 200 μl of CSF rabbit attenuated vaccine virus (purchased from Nanjing Tianbang Biotechnology Co., Ltd.), add 1ml Trizol reagent, shake and mix, let it stand for 10 minutes, add 200 μl of chloroform, shake vigorously, centrifuge at 12000rpm 4°C for 10 minutes, carefully pipette To the supernatant, add an equal volume of isopropanol and mix well, place at -20°C for 2h, centrifuge a...
Embodiment 2
[0041] Example 2 Construction of recombinant expression strain BL21-△E2
[0042] 2.1 Transformation of competent Escherichia coli BL-21
[0043] In Example 1, the correct recombinant plasmid pET32a-e2 was sequenced and transformed into Escherichia coli BL21 (DE3) (purchased from Beijing Quanshijin Biotechnology Co., Ltd.) to obtain the recombinant expression strain BL21-△E2. At the same time, the empty plasmid pET-32a Transform competent Escherichia coli BL-21 in the same way.
[0044] 2.2 Induced expression of recombinant protein
[0045] Single colonies of recombinant expression strain BL21-△E2 and Escherichia coli BL21 containing empty plasmid were picked and inoculated in LB liquid medium containing ampicillin, and cultured overnight at 37°C with shaking.
[0046] (1) Inoculate 10 μL of the overnight cultured bacterial solution into 3 ml LB liquid medium containing ampicillin (50 μg / ml), shake and culture at 200 rpm at 37°C for about 3 hours, and make the OD 600 When it...
Embodiment 3
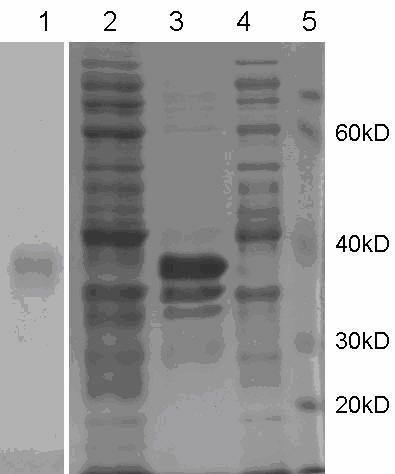
[0053] Example 3 Mass expression, purification and antigenic identification of recombinant protein
[0054] 3.1 Mass expression and purification of recombinant protein
[0055] Pick the positive colonies of the recombinant expression strain BL21-△E2 in 2.2, inoculate in 200ml LB, induce expression according to the induction expression conditions in Example 2, collect the bacteria by centrifugation, add 5ml of PBS to resuspend the bacteria for every 100ml of bacteria solution, and ultrasonically Centrifuge after lysis, separate the supernatant and inclusion bodies, resuspend the inclusion bodies with an equal volume of Binding buffer, and purify the target protein according to the instructions of GE HisTrap HP affinity purification column ( image 3 ). The protein concentration was determined to be 1.2 mg / mL by spectrophotometer, and stored at -20°C after aliquoting.
[0056] 3.2 Western blot identification of recombinant proteins
[0057] (1) SDS-PAGE;
[0058] (2) Transfe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com