Hybridization chirality stationary phase based on cellulose derivative and preparation method thereof
A cellulose derivative, chiral stationary phase technology, applied in chemical instruments and methods, other chemical processes, etc., can solve the problems of reduced processing capacity of preparative chromatography, limited application, loss of cellulose derivatives, etc., to achieve good chirality Resolution and mechanical strength, high content of cellulose derivatives, easily controlled effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
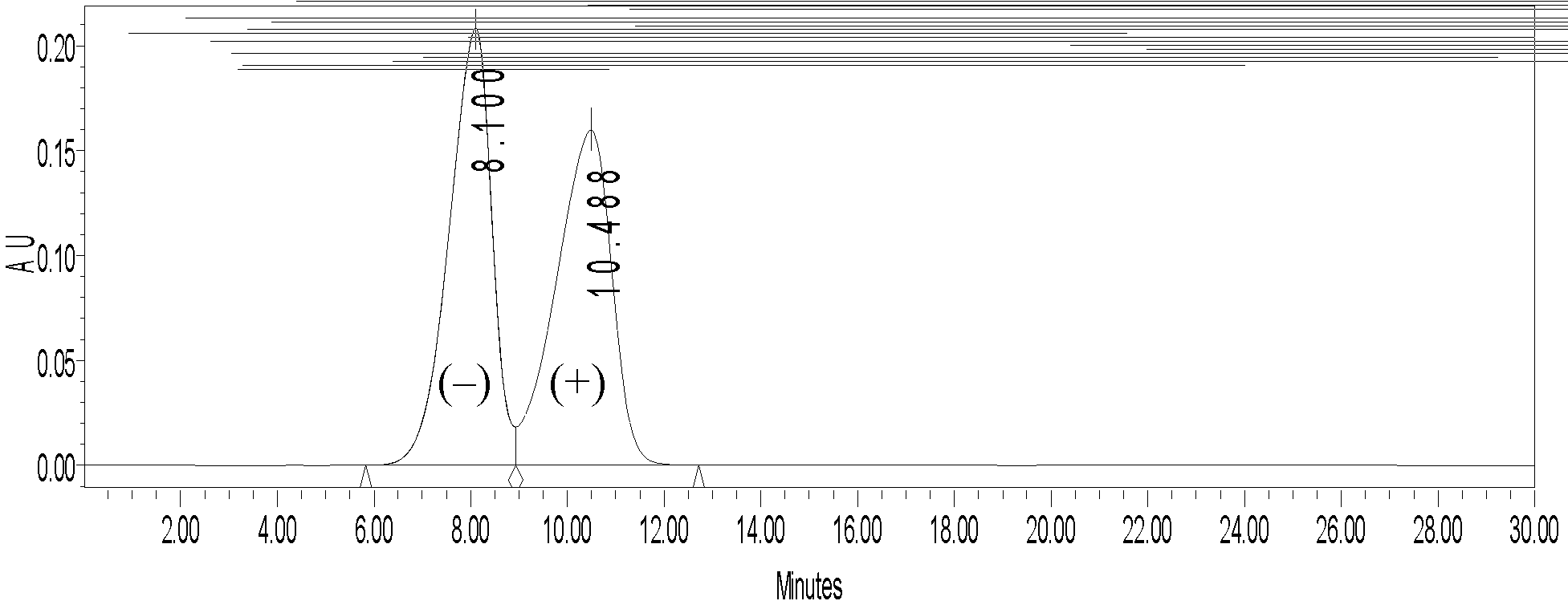
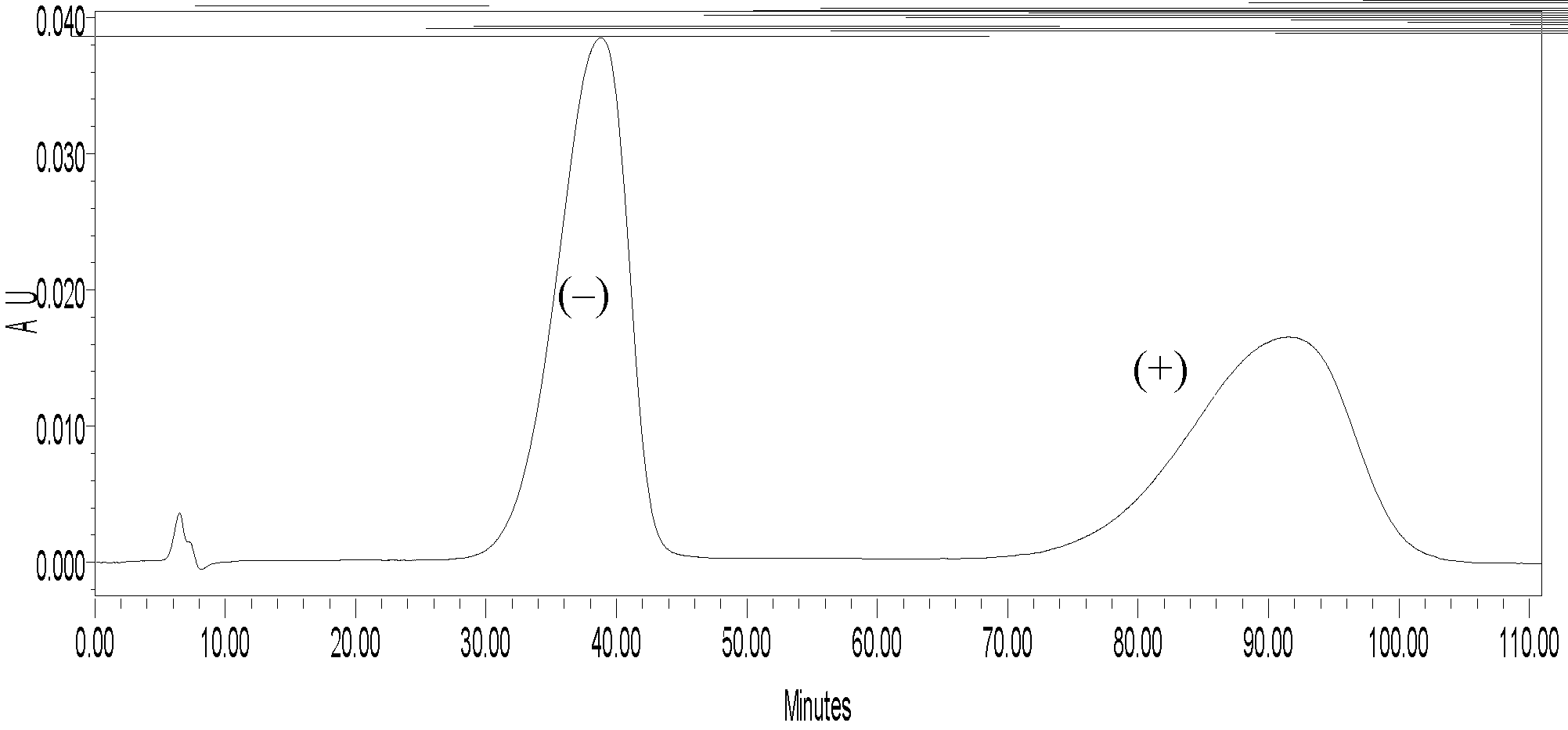
Image
Examples
Embodiment 1
[0030] 1) Synthesis of phenylisocyanate cellulose derivative chiral silane monomer
[0031] Put 0.2 g of cellulose, an appropriate amount of N,N-dimethylacetamide and an appropriate amount of lithium chloride in a three-necked flask, stir and reflux for 1 hour, add 2 ml of anhydrous pyridine after the cellulose is dissolved, and then add 0.62 g (0.005 mol) phenyl isocyanate and 0.05 g (0.0002 mol) tris-(ethoxysilyl) propyl isocyanate mixture, stirred at 80 ° C for 24 hours for derivatization reaction, then precipitated the reaction product with 200 ml of methanol, filtered and dried obtain cellulose derivatives;
[0032] 2) Synthesis of organic-inorganic hybrid silicon spheres
[0033] After dissolving 0.1 g of cellulose derivatives in tetrahydrofuran at 70 °C, add 2 ml of ethyl orthosilicate and 1 ml of water in sequence, add acid to adjust the pH to 2, and cross-link at 60 °C for 2 hours, and the resulting mixture Drop into 500 ml of sodium lauryl sulfate aqueous solution ...
Embodiment 2
[0035] 1) Synthesis of 4-chlorophenylisocyanate cellulose derivative chiral silane monomer
[0036] Put 0.2 g of cellulose, an appropriate amount of N,N-dimethylacetamide, and an appropriate amount of lithium chloride in a three-necked flask, stir and reflux at 70° C. for 1 hour, and add 2 ml of anhydrous pyridine after the cellulose is dissolved. Then add 1.23 grams of 4-chlorophenyl isocyanate (0.008mol) and 0.05 grams (0.0002mol) of tris-(ethoxysilyl)propyl isocyanate mixture, stir at 80°C for derivatization reaction for 24 hours, and then use 200 milliliters of ethanol Precipitating the reaction product, filtering and drying to obtain a cellulose derivative;
[0037] 2) Synthesis of organic-inorganic hybrid silicon spheres
[0038]After dissolving 0.11 g of cellulose derivatives in tetrahydrofuran at 70 °C, add 4 ml of ethyl orthosilicate and 1 ml of water in sequence to adjust the pH to 3, and conduct a crosslinking reaction at 60 °C for 2 hours, and drop the resulting m...
Embodiment 3
[0040] 1) Synthesis of p-methylphenylisocyanate cellulose derivative chiral silane monomer
[0041] Put 0.2 g of cellulose, an appropriate amount of N,N-dimethylacetamide, and an appropriate amount of lithium chloride in a three-necked flask, stir and reflux at 70° C. for 1 hour, and add 2 ml of anhydrous pyridine after the cellulose is dissolved. Then add 0.85 g of p-methylphenyl isocyanate (0.0064 mol) and 0.05 g (0.0002 mol) of a mixture of tris-(ethoxysilyl) propyl isocyanate, stir at 80 ° C for 24 hours, and then use 200 ml Methanol precipitates the reaction product, which is filtered and dried to obtain a cellulose derivative;
[0042] 2) Synthesis of organic-inorganic hybrid silicon spheres
[0043] After dissolving 0.09 g of cellulose derivatives in tetrahydrofuran at 70°C, add 3 ml of ethyl orthosilicate and 1 ml of water in sequence to adjust the pH to 7, and conduct a cross-linking reaction at 60°C for 2 hours, and drop the resulting mixture into 500 milliliters o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com