Method for preparing water-soluble copolymer by using pyrenemethyl acrylate
A technology of pyrene methyl acrylate and water-soluble copolymers, applied in the field of functional polymers, can solve complex synthesis and operation, reduce the detection accuracy of fluorescent probes, and reduce the thermal stability of hybrid double chains of fluorescent probes and targeting molecules and other issues to achieve good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
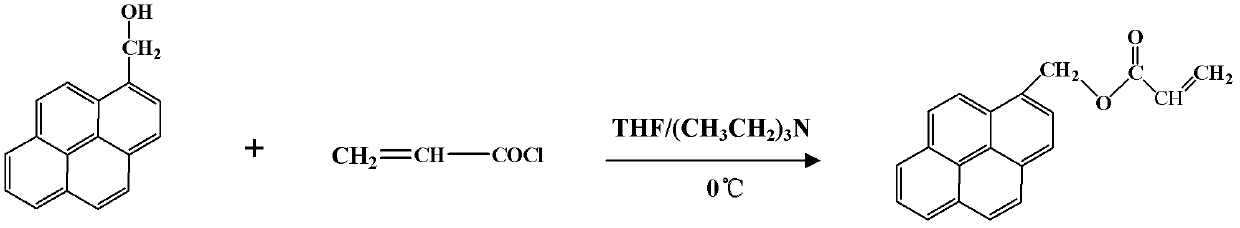
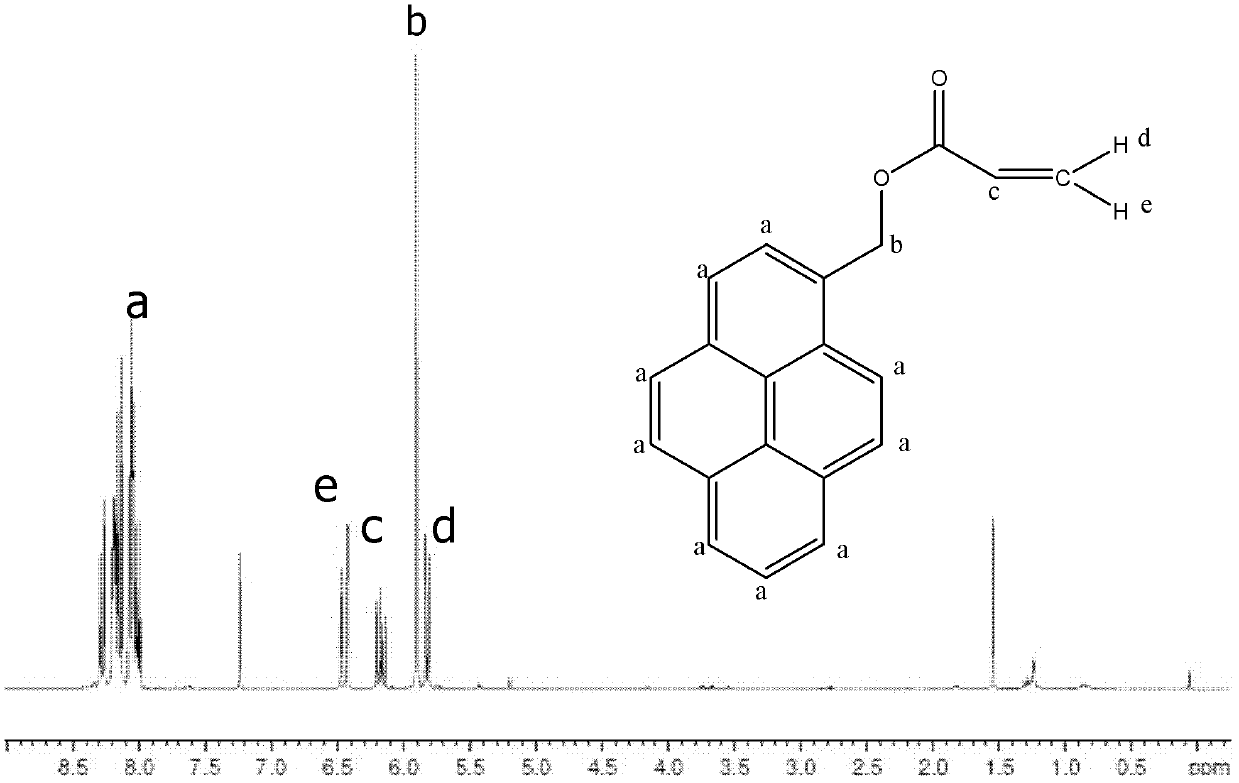
[0018] A 50ml clean three-neck bottle was placed in ice cubes, and N 2 , first add 35ml of dry THF, then add 0.3g of pyrenemethanol, then dropwise add 0.23ml of dry triethylamine, and finally add 0.2ml of acryloyl chloride dissolved in THF slowly with a dropping funnel, so that the reaction gradually Reach room temperature, react 28h. After the reaction, the insoluble precipitate was filtered off, the filtrate was rotary evaporated, and saturated bicarbonate and dichloromethane were added to the filtrate for extraction, saturated sodium bicarbonate was used to neutralize the by-product acrylic acid, and the solution was layered until the organic solution in the lower layer was neutral. Stop extraction. The organic phase was separated, rotary evaporated, and then recrystallized in 95% ethanol to obtain relatively pure pyrene methyl acrylate powder.
example 2
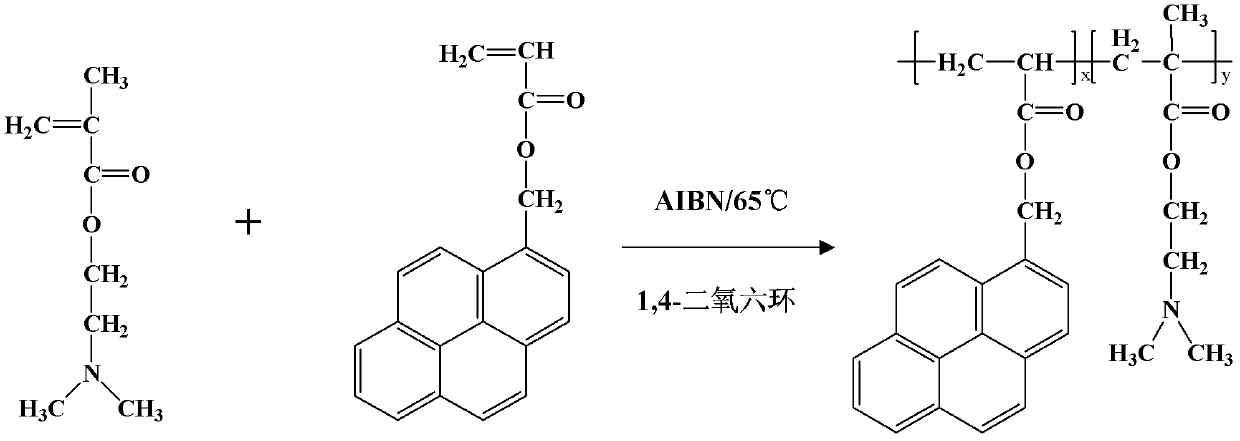
[0020] Add 40ml of dry-treated 1,4-dioxane to a 50ml clean shlenk bottle, and blow N into it 2 , and then add 0.05mmol pyrenemethyl acrylate, 20mmol dimethylaminoethyl methacrylate and 0.1mmol azobisisobutylcyanide (AIBN) (recrystallized) respectively, and react in an oil bath at 60°C for 5h, After reaction finishes, in the sherwood oil that reaction product is added dropwise in 500ml, precipitates three times, because initiator and monomer dissolve in solvent, and polymer is precipitation in solvent, polymer can be separated by this method, Dry overnight in a vacuum oven at 70°C to obtain the final dried product. Elemental analysis and NMR analysis were done on the obtained product. In the elemental analysis, the nitrogen element content is 7.97%, the carbon element content is 57.17%, and the hydrogen element content is 9.16%. The copolymerization ratio x:y=1:54 is calculated. GPC results show: Mn=1941, Mw=7309, Mz=15296, Mz / Mw=2.092786.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com