Nano linear calcium titanate and synthesis method thereof
A synthesis method and technology of calcium titanate, applied in the field of nanowire calcium titanate and the synthesis of such nanowire calcium titanate, can solve the problems of restricting large-scale production, template destruction, inability to reuse, etc., to meet the needs of large-scale production. Large-scale industrial production, excellent ecological biocompatibility, and the effect of avoiding high-temperature solid-phase reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
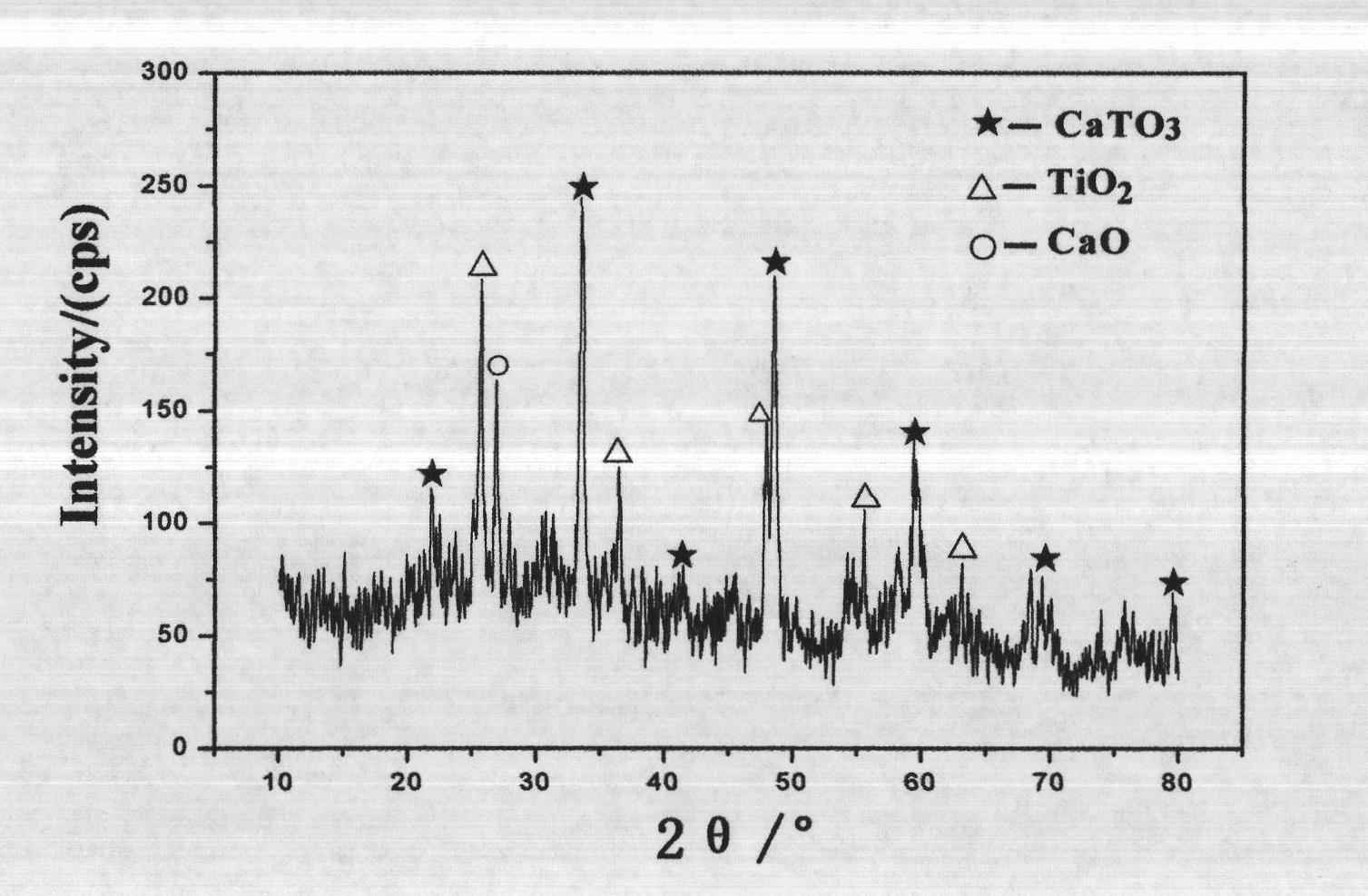
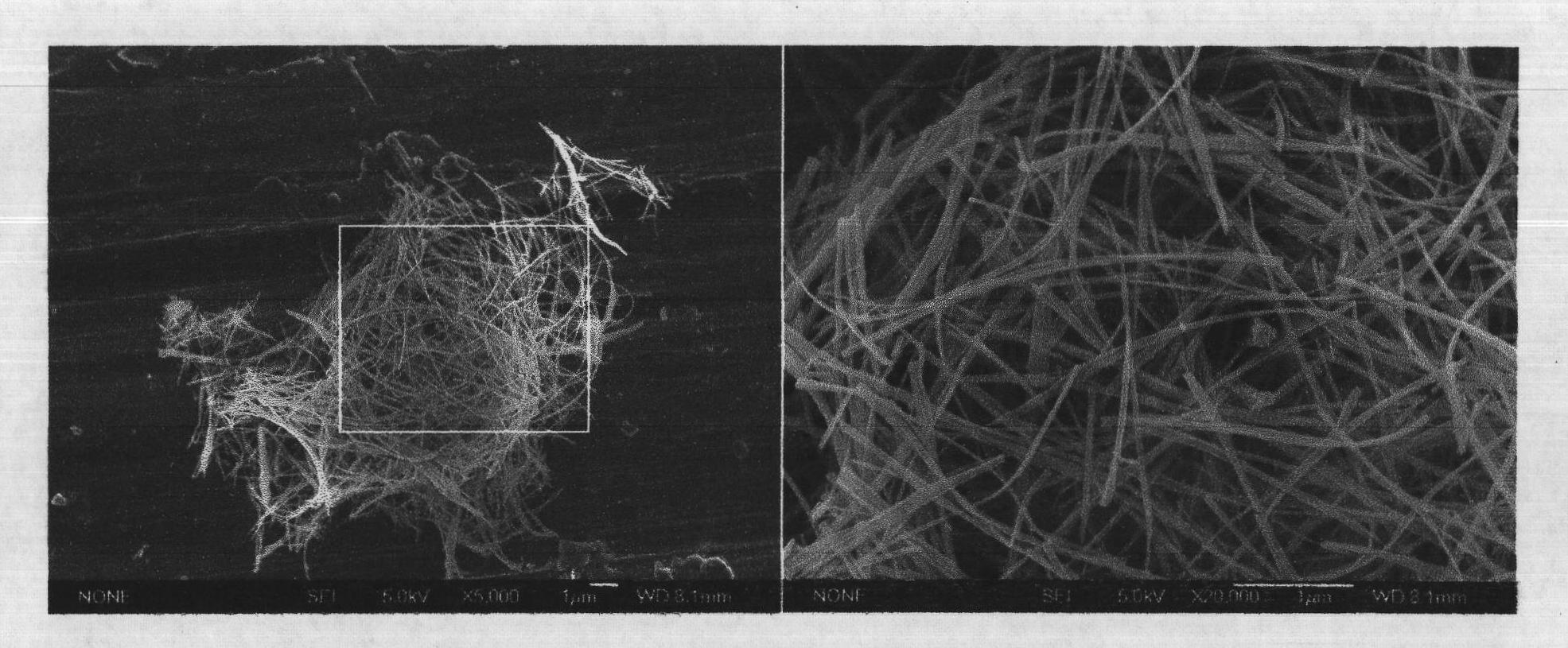
Image
Examples
Embodiment 1
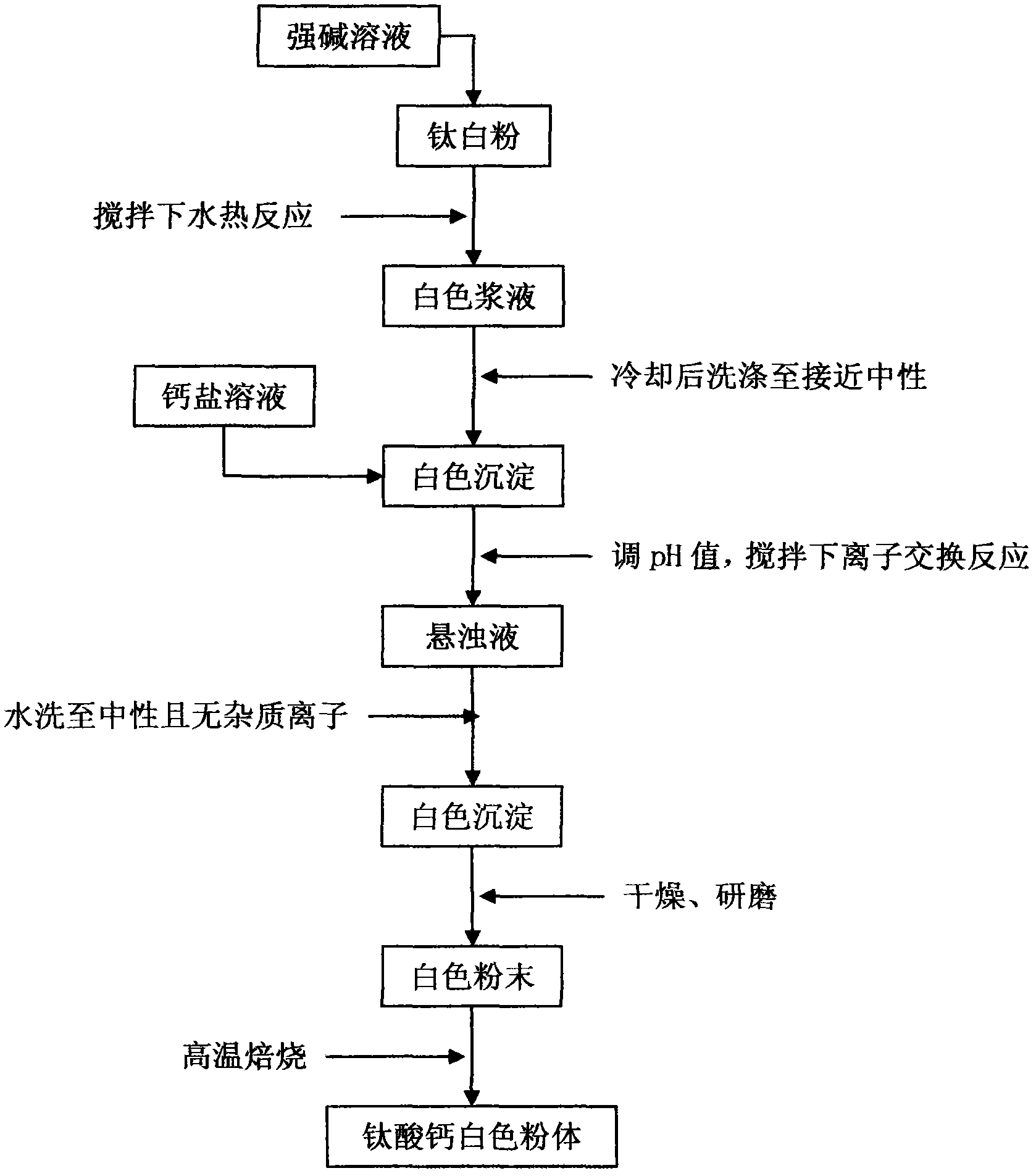
[0029] 1) Preparation of strong base solution: accurately weigh 100 grams of analytically pure sodium hydroxide, dissolve it in 500 mL of deionized water to obtain strong base solution A, and the concentration of substances in strong base solution A is 5 mol / L;
[0030] 2) Hydrothermal reaction: Accurately weigh 0.8 g of analytically pure titanium dioxide, add 30 mL of strong alkali solution A and stir evenly, transfer to a stainless steel reaction kettle, and magnetically stir the hydrothermal reaction at 150°C for 15 hours , after cooling, wash until nearly neutral to obtain white precipitate B;
[0031] 3) Preparation of soluble calcium salt solution: accurately weigh 25 grams of analytically pure anhydrous calcium chloride, dissolve in 500 mL of deionized water to obtain soluble calcium salt solution C, and the mass concentration of soluble calcium salt solution C is 50 g / L;
[0032] 4) Ion exchange reaction: Take 35 mL of soluble calcium salt solution C, add it to the abo...
Embodiment 2
[0035] 1) Preparation of strong alkali solution: accurately weigh 150 grams of analytically pure sodium hydroxide, dissolve it in 250 mL of deionized water to obtain strong alkali solution A, and the concentration of substances in strong alkali solution A is 15 mol / L;
[0036] 2) Hydrothermal reaction: Accurately weigh 0.8 g of analytically pure titanium dioxide, add 30 mL of strong alkali solution A and stir evenly, then transfer to a stainless steel reaction kettle, and magnetically stir the hydrothermal reaction at 250°C for 15 hours , after cooling, wash until nearly neutral to obtain white precipitate B;
[0037] 3) Preparation of soluble calcium salt solution: accurately weigh 5 grams of analytically pure anhydrous calcium chloride, dissolve in 500 mL of deionized water to obtain soluble calcium salt solution C, and the mass concentration of soluble calcium salt solution C is 10 g / L;
[0038] 4) Ion exchange reaction: Measure 120 mL of soluble calcium salt solution C, ad...
Embodiment 3
[0041] 1) Preparation of strong base solution: accurately weigh 200 grams of analytically pure sodium hydroxide, dissolve it in 500 mL of deionized water to obtain strong base solution A, and the concentration of substances in strong base solution A is 10 mol / L;
[0042] 2) Hydrothermal reaction: Accurately weigh 0.8 g of analytically pure titanium dioxide, add 30 mL of strong alkali solution A and stir evenly, then transfer to a stainless steel reaction kettle, and magnetically stir the hydrothermal reaction at 180°C for 24 hours , after cooling, wash until nearly neutral to obtain white precipitate B;
[0043]3) Preparation of soluble calcium salt solution: accurately weigh 25 grams of analytically pure anhydrous calcium chloride, dissolve in 500 mL of deionized water to obtain soluble calcium salt solution C, and the mass concentration of soluble calcium salt solution C is 50 g / L;
[0044] 4) Ion exchange reaction: Take 35mL of soluble calcium salt solution C, add it to the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com