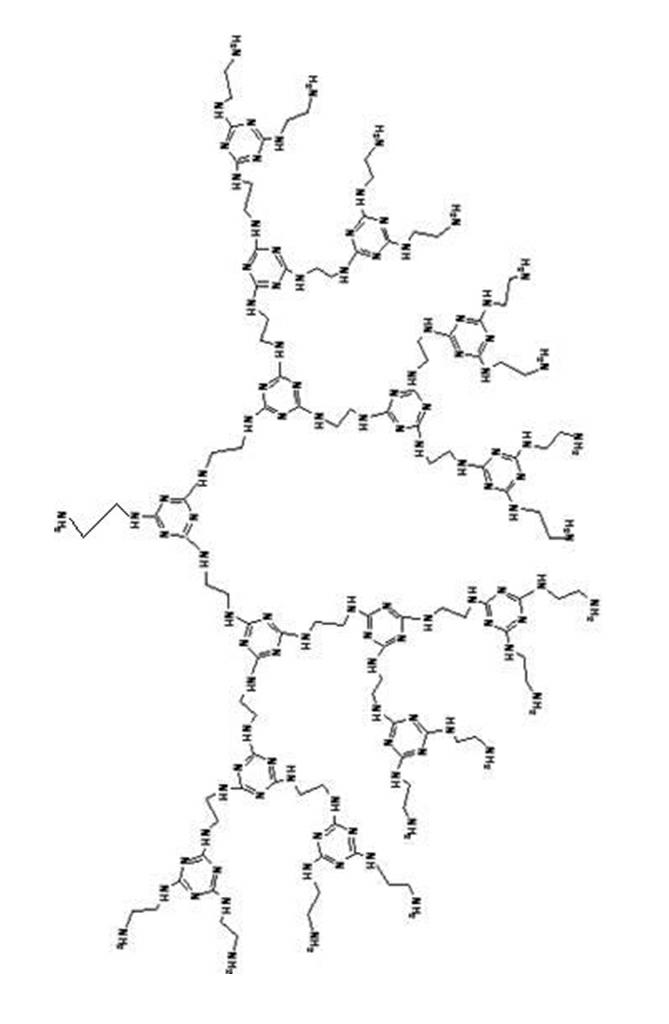
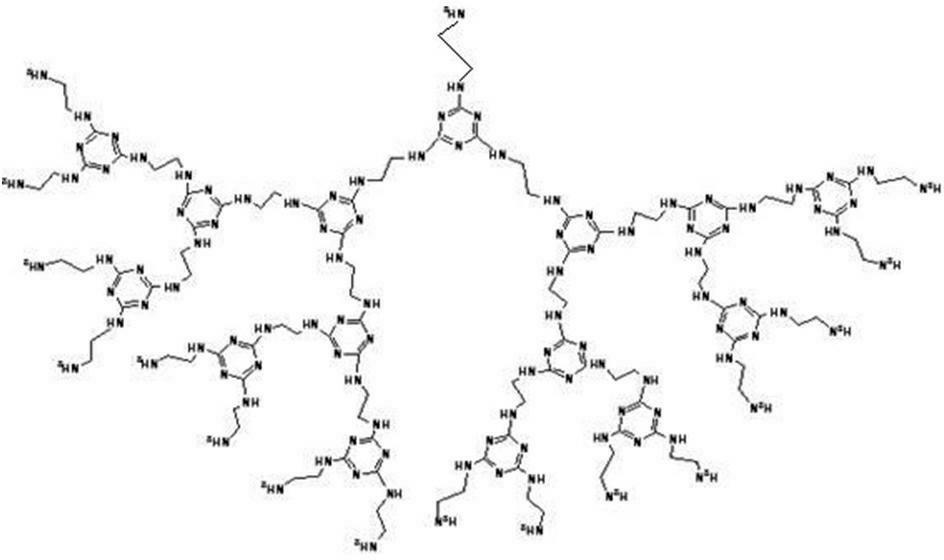
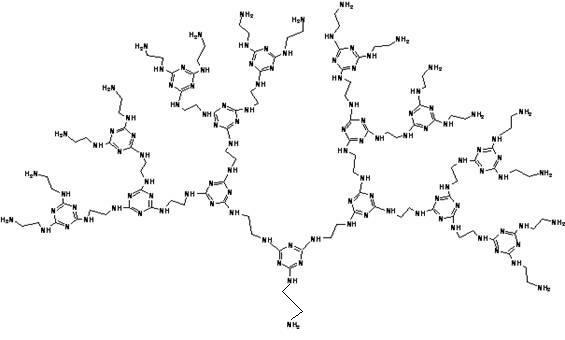
Amino end-group triazine ring tree-shaped macromolecular adsorbent and preparation method thereof
A macromolecule-like, s-triazine technology, applied in the field of amino-terminated s-triazine ring dendrimer adsorbent and its preparation, achieving the effects of small usage amount, low cost and wide usage range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The specific preparation method of the amino-terminated s-triazine ring dendrimer adsorbent is as follows:
[0029] Step 1: Under the condition of an ice-water bath, mix cyanuric chloride and acetone at a molar ratio of 1:10, then slowly add ethylenediamine, the molar ratio of ethylenediamine to cyanuric chloride is 2.0:1, Stir the reaction at 0°C for 1 hour;
[0030] Step 2: Add a mass fraction of 35% sodium hydroxide solution to the reaction mixture obtained in step 1, the molar ratio of sodium hydroxide to ethylenediamine is 0.8:1, stir and react at 20°C for 6 hours; Stirring reaction at ℃ for 6 hours;
[0031] Step 3: The filter cake obtained by suction filtration of the mixture obtained in step 2 was extracted and washed three times with 200 mL of acetone to remove unreacted cyanuric chloride, then washed three times with deionized water, and dried at 70 ° C to obtain amino-terminated Triazine ring dendrimer adsorbent.
Embodiment 2
[0033] The specific preparation method of the amino-terminated s-triazine ring dendrimer adsorbent is as follows:
[0034] Step 1: Under the condition of an ice-water bath, mix cyanuric chloride and acetone at a molar ratio of 1:15, then slowly add ethylenediamine, the molar ratio of ethylenediamine to cyanuric chloride is 2.5:1, Stir the reaction at 0°C for 3 hours;
[0035] Step 2: add a mass fraction of 35% sodium bicarbonate solution to the mixture obtained in step 1 reaction, the molar ratio of sodium bicarbonate to ethylenediamine is 1:1, stir and react at 25°C for 5 hours; Stir the reaction at ℃ for 5 hours;
[0036] Step 3: The filter cake obtained by suction filtration of the mixture obtained in step 2 was extracted and washed three times with 200 mL of acetone to remove unreacted cyanuric chloride, then washed three times with deionized water, and dried at 70 ° C to obtain amino-terminated Triazine ring dendrimer adsorbent.
Embodiment 3
[0038] Step 1: Under the condition of an ice-water bath, mix cyanuric chloride and acetone at a molar ratio of 1:20, then slowly add ethylenediamine, the molar ratio of ethylenediamine and cyanuric chloride is 3:1, Stir the reaction at 0°C for 5 hours;
[0039] Step 2: Add a sodium carbonate solution with a mass fraction of 35% to the mixture obtained in the reaction in step 1. The molar ratio of sodium carbonate to ethylenediamine is 1.2:1, and stir and react at 35°C for 3 hours; then at 90°C Stir the reaction for 3 hours;
[0040] Step 3: The filter cake obtained by suction filtration of the mixture obtained in step 2 was extracted and washed three times with 200 mL of acetone to remove unreacted cyanuric chloride, then washed three times with deionized water, and dried at 70 ° C to obtain amino-terminated Triazine ring dendrimer adsorbent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com