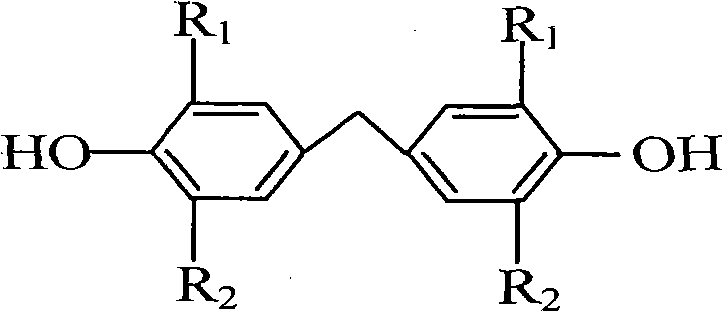
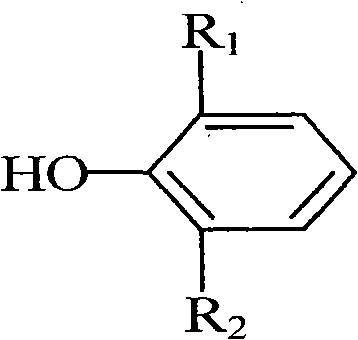
Clean production method for preparing bisphenol antioxidant
A clean production and antioxidant technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of increased labor consumption and power consumption, limited number of repetitions, unfavorable environmental protection, etc., and achieve economical reaction Time, easy procurement, and the effect of protecting water resources and the environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] In a 500 ml four-neck flask equipped with electric stirring, a thermometer, a reflux condenser, and a nitrogen replacement device, add 100 grams (0.485 moles) of 2,6-di-tert-butylphenol, 150 milliliters of industrial ethanol (concentration 97%), Add 0.4 g of catalyst sodium hydroxide, replace with nitrogen, start stirring, raise the temperature to 65°C, start to slowly add 20.0 g of formaldehyde solution (37% formaldehyde concentration, 0.246 moles of formaldehyde) dropwise, and finish the dropwise addition in about 30 minutes. The temperature of the material is 75-85°C, keep the reflux reaction, after observing a large number of crystals, cool down to 50-60°C, replace with nitrogen, add 50 ml of ethanol, replace with nitrogen again, raise the temperature to 75-85°C, and continue the reaction After 20 minutes, after the reaction was replaced by nitrogen, 0.64 g of citric acid was added for neutralization. The neutralization temperature was 80-85°C, and the neutralization...
Embodiment 2
[0032] In a 500 ml four-necked flask equipped with electric stirring, a thermometer, a reflux condenser, and a nitrogen replacement device, add 100 grams (0.485 moles) of 2,6-di-tert-butylphenol, and 150 milliliters of industrial ethanol (recovered from Example 1) Ethanol, concentration 95%), add 0.5 g of catalyst potassium hydroxide, replace with nitrogen, start stirring, heat up to 80 ° C, start to slowly add 20.5 g of formaldehyde aqueous solution (formaldehyde concentration 37%, containing 0.253 moles of formaldehyde), about 40 minutes After the dropwise addition, control the temperature of the material in the flask to 80-85°C, and keep the reflux reaction. After observing a large amount of crystallization, cool down to 50-60°C, replace with nitrogen, add 50 ml of ethanol, replace with nitrogen again, and heat up to 80-85°C, continue to react for 20 minutes, add 0.535 g of glacial acetic acid for neutralization after the reaction is completed, and replace with nitrogen. The...
Embodiment 3
[0035]In a 500 ml four-neck flask equipped with electric stirring, a thermometer, a reflux condenser, and a nitrogen replacement device, add 100 grams (0.485 moles) of 2,6-di-tert-butylphenol, 150 ml of industrial isopropanol (concentration 95%) ), add 0.4 g of catalyst sodium hydroxide, replace with nitrogen, start stirring, heat up to 80°C, slowly add 19.0 g of formaldehyde solution (formaldehyde concentration 40%, containing 0.253 moles of formaldehyde) dropwise, and the dropwise addition ends in about 30 minutes. The temperature of the internal material is 80-90°C, keep the reflux reaction, after observing a large amount of crystallization, cool down to 50-60°C, replace with nitrogen, add 50 ml of isopropanol, replace with nitrogen again, and heat up to 80-90°C , continue to react for 20 minutes, add 0.64 g of citric acid for neutralization after the reaction is replaced by nitrogen, the neutralization temperature is 80-85°C, and the neutralization time is 10 minutes. ; an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com