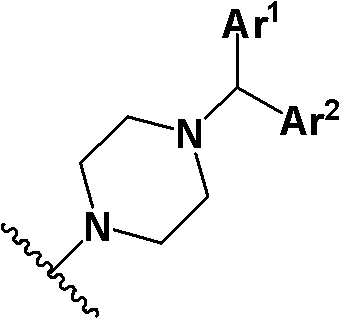
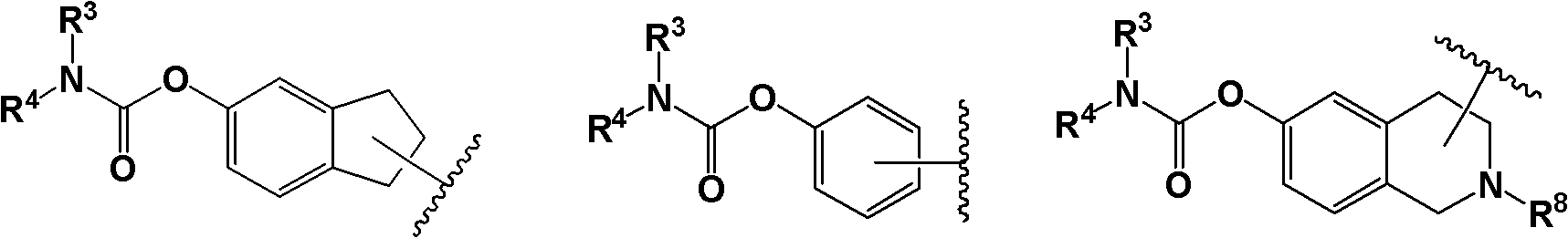
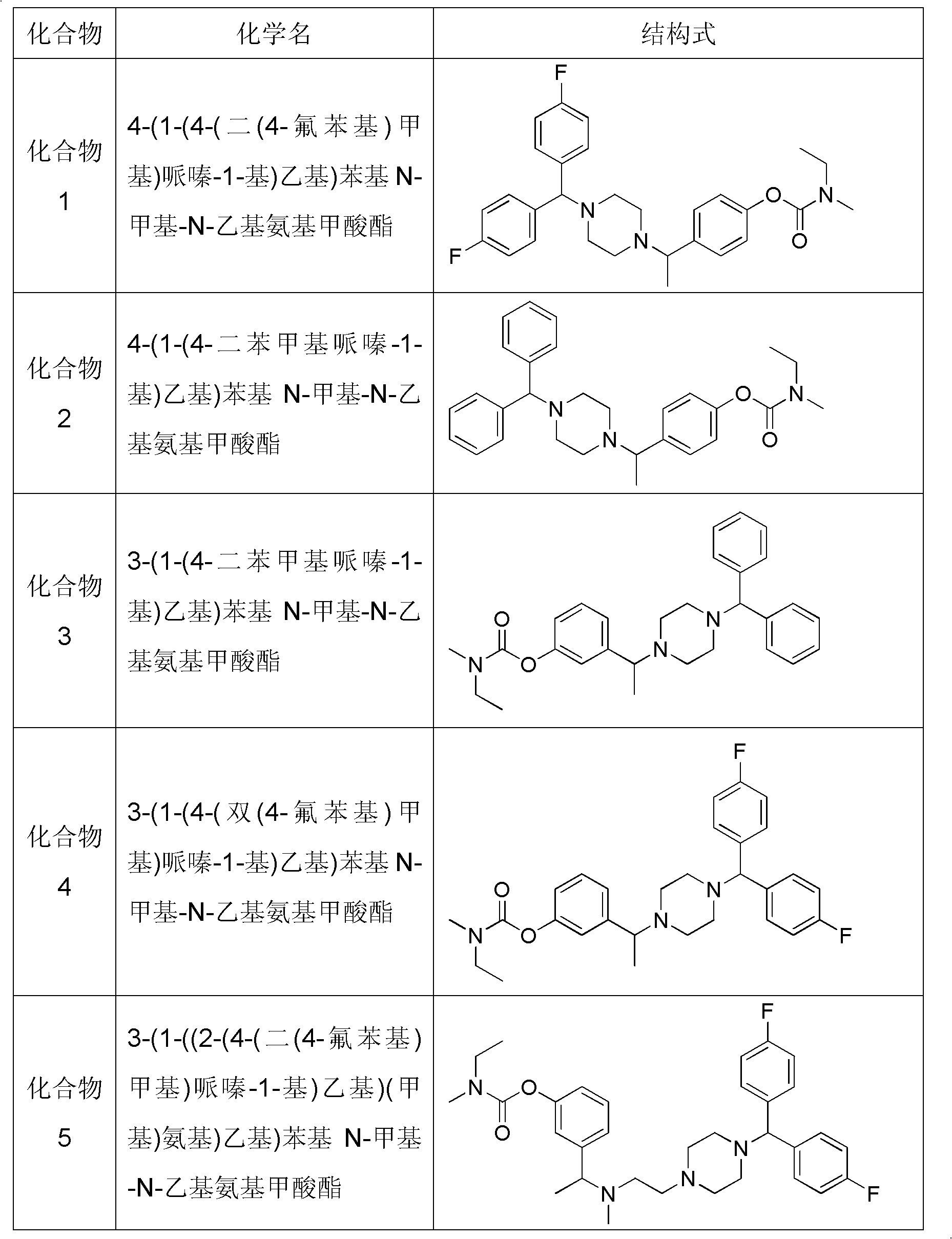
Piperazine compound and application thereof
A compound and selected technology, applied in organic chemistry, drug combination, medical preparations containing active ingredients, etc., can solve problems affecting the body's cognition, memory, acetylcholine deficiency, and failure of nerve signal transmission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1 prepares compound 1 provided by the invention
[0051] The preparation process is as follows:
[0052]
[0053] At room temperature, 62.7g of anhydrous piperazine about 72.9mmol in 100ml of toluene, add 5.7g of K 2 CO 3 About 41.8mmol of solid powder and about 20.9mmol of 3.1g of catalytic NaI were heated and stirred until the solid was dissolved, and about 20.9mmol of 5.0g of 4,4'-difluorodiphenylchloromethane was added, and the system was heated and refluxed for 8 hours. The reaction was detected by TLC and the reaction was complete After cooling, the reaction system was filtered, and the filtrate was concentrated to obtain an oil, which was directly purified by silica gel column chromatography, using petroleum ether: ethyl acetate = 1: 1 ~ 1: 2 ~ pure ethyl acetate (1% triethylamine) Gradient elution to obtain the oily product S 1 4.87g, yield 81.9%. ESI-MS[M+H] + =289.1
[0054]Weigh 10.0g of p-hydroxyacetophenone about 7.4mmol, 8.9g of N-meth...
Embodiment 2
[0059] Embodiment 2 prepares compound 2 provided by the invention
[0060] The preparation process is as follows
[0061]
[0062] Put 8.6g of anhydrous piperazine about 100mmol in 100ml of acetonitrile, add K 2 CO 3 Solid powder 13.8g is about 50mmol and catalytic amount NaI 1.5g is about 10mmol, heat and stir to dissolve the solid, add about 12.3g of diphenyl bromide about 50mmol, the system is heated and refluxed for 8h, TLC detects the reaction, after the reaction is complete, cool , filter the reaction system, and concentrate the filtrate to obtain an oil, which is directly purified by silica gel column chromatography, using petroleum ether: ethyl acetate = 1: 1 ~ 1: 2 (1% triethylamine), pure ethyl acetate (1% Triethylamine) gradient elution, obtain oil product S 2 8.17g, yield 64.8%. ESI-MS[M+H] + =253.2
[0063] Put 110mg S2 about 0.43mmol and 100mg S6 about 0.41mmol into the reaction bottle, add 130mg K 2 CO 3 The powder is about 0.94mmol and 15ml of aceto...
Embodiment 3
[0065] Embodiment 3 prepares compound 3 provided by the invention
[0066] The preparation process is as follows:
[0067]
[0068] Get 8.0g of 3-hydroxyacetophenone to be about 58.8mmol, 7.2g of N-methyl-N-ethylformyl chloride to be about 59.5mmol and K 2 CO 3 Add 16.5g of solid powder (about 122.2mmol) into the system, and add 150ml of acetone, reflux reaction, TLC detection After the reaction is completed, cool, filter the reaction solution, condense the filtrate and evaporate to dryness to obtain the oil S 6 13.3 g, yield 87.5%. ESI-MS[M+H] + =222.1
[0069] Will S 6 13.3g about 60.2mmol was dissolved in 150ml of methanol, slowly added NaBH under ice bath 4 4.1g is about 108.5mmol. There are bubbles in the system. Warm up to room temperature and stir overnight. TLC detects the reaction. After the reaction is complete, evaporate the reaction solution, add distilled water, and extract with ethyl acetate. The organic layer is washed with saturated saline. The organ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com