Preparation method of dehydroisoandrosterone
A technology for dehydroepiandrosterone and ketal compounds, which is applied in the field of preparation of dehydroepiandrosterone, can solve the problems of only 70% yield, temperature sensitivity, and difficulty in control, and achieves simple post-processing process and easy reaction conditions. Controlled, high-yield effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
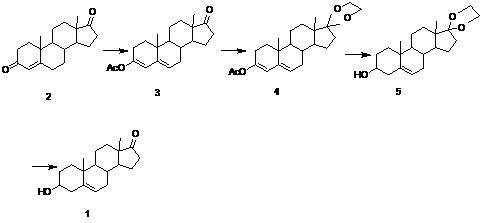
Examples
Embodiment 1
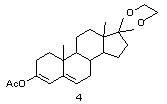
[0035] Esterification reaction
[0036] Preparation of esterified product 3: Add 10 grams of compound 2 and 350 ml of acetic anhydride into the reactor under nitrogen protection, dissolve the system under stirring, then add 6 grams of p-toluenesulfonic acid at 20° C. ℃-25℃ for 5 hours, TLC detects that about 1%-2% of the raw materials are left, then control the temperature below 5℃ and slowly pour the system into 200 ml of ice water, stir at 0-5℃ for 2 hours, 0- Stand at 5°C for 2 hours, filter, wash with a large amount of water until neutral, and dry in vacuum at 40°C for 24 hours to obtain 10.74 g of white or off-white solid, with a mass yield of 107.4%.
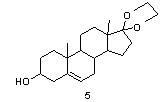
[0037] Ketal reaction
[0038] Preparation of ketal 4: Add 20 grams of esterified product, 100 ml of ethylene glycol, and 100 ml of triethyl orthoformate into the reaction flask in sequence under nitrogen protection, and stir at 20°C for 10 minutes, and the system becomes turbid. Finally, add 0.13 g of p-toluenesulfonic ...
Embodiment 2
[0044] Preparation of esterified product 3: Add 10 grams of compound 2 and 150 ml of acetic anhydride into the reactor under nitrogen protection, dissolve the system under stirring, then add 7 grams of p-toluenesulfonic acid at 20°C under temperature control, and add 7 grams of p-toluenesulfonic acid at 20 ℃-25℃ for 5 hours, TLC detects that about 1%-2% of the raw materials are left, then control the temperature below 5℃, slowly pour the system into 200 ml of ice water, stir at 0-5℃ for 2 hours, 0- Stand at 5°C for 2 hours, filter, wash with a large amount of water until neutral, and dry in vacuum at 40°C for 24 hours to obtain 10.87 g of white or off-white solid, with a mass yield of 108.7%.
[0045] Preparation of ketal 4: Add 19.95 g of esterified product, 60 ml of ethylene glycol, and 70 ml of triethyl orthoformate into the reaction flask in sequence under nitrogen protection, and stir at 20°C for 10 minutes, and the system becomes turbid. Finally, add 0.2 gram of p-toluen...
Embodiment 3
[0049] Preparation of esterified product 3: Add 10 grams of compound 2 and 400 ml of acetic anhydride into the reactor under nitrogen protection, dissolve the system under stirring, then add 4.5 grams of p-toluenesulfonic acid at 20° C. ℃-25℃ for 5 hours, TLC detects that about 1%-2% of the raw material remains, then control the temperature below 5℃ and slowly pour the system into 200 ml of ice water, stir at 0-5℃ for 2 hours, 0-5℃ Stand at 5°C for 2 hours, filter, wash with a large amount of water until neutral, and dry under vacuum at 40°C for 24 hours to obtain 10.67 g of white or off-white solid, with a mass yield of 106.7%.
[0050] Preparation of ketal 4: Add 19.95 g of esterified product, 130 ml of ethylene glycol, and 90 ml of trimethyl orthoformate into the reaction flask in sequence under nitrogen protection, and stir at 20°C for 10 minutes, and the system becomes turbid. Finally, add 4 grams of p-toluenesulfonic acid, keep it warm at 20°C for an hour, then add 1 gra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com