Oligopeptide-based pH-sensitive amphoteric ion and application thereof in medicament
A drug and matrix technology, applied in the field of oligopeptide pH-sensitive zwitterionic lipids and its preparation, can solve problems such as carrier damage and drug leakage, and achieve good blood compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
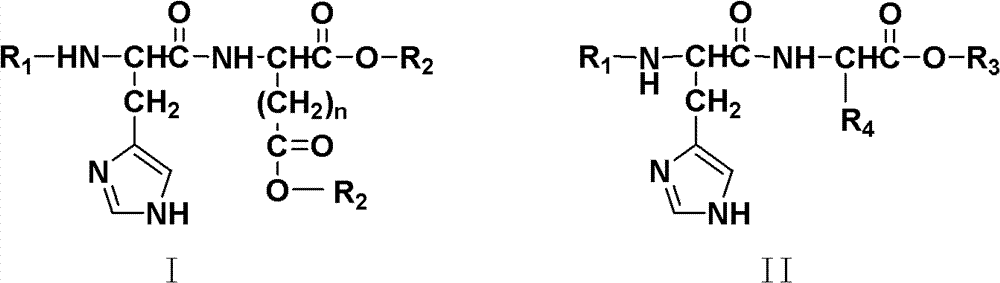
[0044] Preparation of 1,5-eicosanol-L-glutamic acid-histidine-succinic anhydride
[0045] Glutamic acid (11.8 g, 80.2 mmol) and p-toluenesulfonic acid (18.3 g, 96.2 mmol) were dissolved in 350 mL of toluene, and refluxed for 1 h. Add n-eicosanol (52.8g, 176.7mol) and reflux for 12h. After the reaction, the toluene was distilled off under reduced pressure. The concentrate was dissolved in an appropriate amount of dichloromethane, washed with 5% sodium bicarbonate solution (100mL×2), washed with water (100mL×1), the organic layer was dried over anhydrous sodium sulfate, concentrated, and recrystallized from methanol to obtain a white powdery solid 1 , 5-Eicosanol-L-glutamic acid (EI 2 -Glu, 32 g, 56.2%). Boc-L-His(Tos)-OH (6.9g, 16.8mmol), N,N-dicyclohexylcarbodiimide (DCC, 10.3g, 49.9mmol) and N-hydroxysuccinimide (NHS , 2.9g, 25.2mmol) was dissolved in 100mL N, N-dimethylformamide (DMF), stirred at room temperature for 3h; EI 2 -Glu (12g, 16.9mmol) was added to the above ...
Embodiment 2
[0049] 1,5-n-octadecyl alcohol-L-glutamic acid-histidine-citrafuric anhydride (SA 2 -Glu-His-CTA) preparation
[0050] Glutamic acid (11.8 g, 80.2 mmol) and p-toluenesulfonic acid (18.3 g, 96.2 mmol) were dissolved in 350 mL of toluene, and refluxed for 1 h. Add octadecyl alcohol (47.8g, 176.7mol) and reflux for 12h. After the reaction, the toluene was distilled off under reduced pressure. The concentrate was dissolved in an appropriate amount of dichloromethane, washed with 5% sodium bicarbonate solution (100mL×2), washed with water (100mL×1), the organic layer was dried over anhydrous sodium sulfate, concentrated, and recrystallized from methanol to obtain a white powdery solid 1 , 5-octadecyl-L-glutamic acid (SA 2 -Glu, 29 g, 55.4%). Boc-L-His(Tos)-OH (6.9g, 16.8mmol), DCC (10.3g, 49.9mmol) and NHS (2.9g, 25.2mmol) were dissolved in 100mLDMF, stirred at room temperature for 3h; 2 -Glu (11 g, 16.9 mmol) was added to the above mixed solution and stirred at room temperatu...
Embodiment 3
[0054] Preparation of 1,5-n-dodecyl alcohol-L-glutamic acid-histidine-hexahydrophthalic anhydride
[0055] Glutamic acid (11.8 g, 80.2 mmol) and p-toluenesulfonic acid (18.3 g, 96.2 mmol) were dissolved in 350 mL of toluene, and refluxed for 1 h. Add n-dodecyl alcohol (3.3g, 176.7mol) and reflux for 12h. After the reaction, the toluene was distilled off under reduced pressure. The concentrate was dissolved in an appropriate amount of dichloromethane, washed with 5% sodium bicarbonate solution (100mL×2), washed with water (100mL×1), the organic layer was dried over anhydrous sodium sulfate, concentrated, and recrystallized from methanol to obtain a white powdery solid 1 , 5-n-dodecyl-L-glutamic acid (DO 2 -Glu, 17 g, 44.1%). Boc-L-His(Tos)-OH (6.9g, 16.8mmol), DCC (10.3g, 49.9mmol) and NHS (2.9g, 25.2mmol) were dissolved in 100mLDMF, stirred at room temperature for 3h; 2 -Glu (8.1 g, 16.9 mmol) was added to the above mixed solution and stirred at room temperature for 12 h. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| surface potential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com