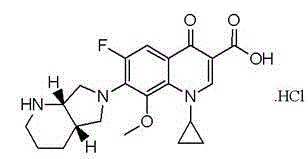
Stable moxifloxacin hydrochloride compound and preparation method thereof
A technology of moxifloxacin hydrochloride and its compound, which is applied in the field of pharmaceuticals in the field of medicine, can solve the problems of unfavorable industrial production, low adoption yield, cumbersome operation, etc., and achieve the effect of simplified operation, simple operation, and simplified process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
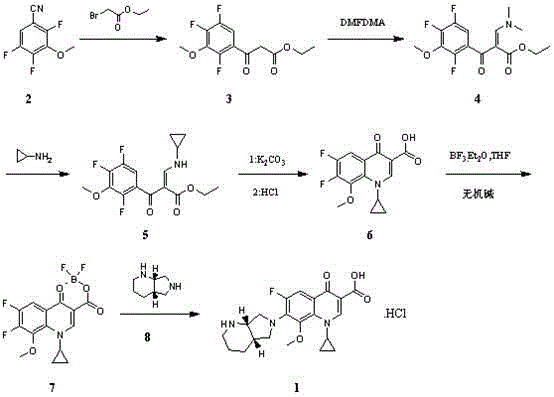
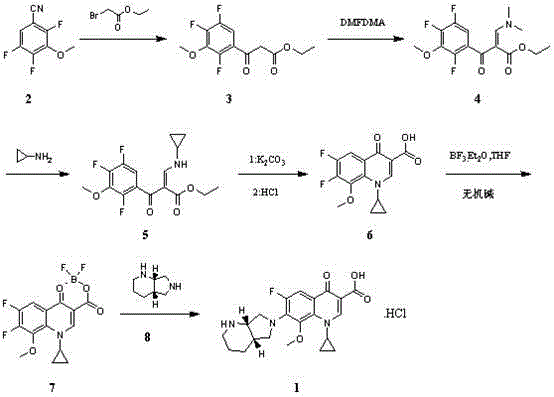
[0083] Add 187g of 3-methoxy-2,4,5-trifluorobenzonitrile, 97.5g of zinc and 1L of tetrahydrofuran into a 3L three-necked flask, add 8.6g of p-toluenesulfonic acid under stirring, and stir at room temperature for 0.5h. Heated to reflux, and slowly added dropwise 217.1 g of ethyl bromoacetate. React under reflux for 2 hours, cool to room temperature, add 500mL 6M hydrochloric acid and 100mL water successively, stir for 2h, cool to 5°C, a large amount of solid precipitates, filter, rinse with 100mL ethanol, and blow dry at 40°C to obtain 223.5g3- Methoxy-2,4,5-trifluorobenzoyl ethyl acetate, yield 80.9%, purity 98.5%.
Embodiment 2
[0085] Add 220g of ethyl 3-methoxy-2,4,5-trifluorobenzoylacetate, 1.1L of methanol and 142.8g of N,N-dimethylformamide dimethyl acetal into a 3L three-necked flask, and react at room temperature for 2h. TLC showed that the reaction was complete, and concentrated under reduced pressure to obtain 270 g of ethyl 3-dimethylamine-2-(3-methoxy-2,4,5-trifluorobenzoyl)acrylate. Dissolve 270g of ethyl 3-dimethylamine-2-(3-methoxy-2,4,5-trifluorobenzoyl)acrylate in 1.35L of dichloromethane for later use.
Embodiment 3
[0087] Add 54.7g cyclopropylamine and 1L dichloromethane into a 5L three-necked flask, add 3-dimethylamine-2-(3-methoxy-2,4,5-trifluorobenzoyl)acrylic acid dropwise at room temperature The dichloromethane solution of ethyl ester was stirred for 1 h, and the reaction was stopped as detected by TLC that the reaction was complete. Add 2L of water to the reaction solution, stir for 20 minutes, let stand for liquid separation, wash the organic phase with 1L of 10% aqueous sodium bicarbonate solution, and separate the liquids. The aqueous phases were combined, and the aqueous phase was extracted with 1L of dichloromethane, the organic phases were combined, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to dryness to obtain 280.5g of 3-cyclopropylamine-2-(3-methoxy-2,4, 5-Trifluorobenzoyl) ethyl acrylate. Dissolve ethyl 3-cyclopropylamine-2-(3-methoxy-2,4,5-trifluorobenzoyl)acrylate in 3 L of DMSO for later use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com