Method for detecting trypsin using unmarked fluorescence
A trypsin and fluorescence detection technology, which is applied in the interdisciplinary fields of analytical chemistry, biology, and materials, can solve unseen problems and achieve the effects of simple preparation, short detection time, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
[0029] Ⅰ Preparation of organic solution of fluorescent probe:
[0030] Using pyrene as a fluorescent probe, dissolve pyrene in acetone and prepare a concentration of 10 in a 10 mL volumetric flask. -2 mol L -1 Pyrene in acetone, further diluted with acetone to 10 -5 mol L -1 ;
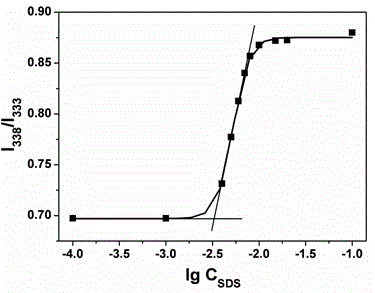
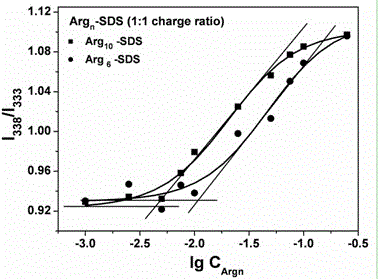
[0031] Ⅱ Determination of critical micelle concentration CMC of surfactant:
[0032] Dissolve sodium dodecyl sulfate SDS in a phosphate buffer solution with a concentration of 10 mM and pH 8.0, and prepare 10 in a 10 mL volumetric flask -2 mol L -1 The solution is further diluted with buffer solution to obtain a concentration range of 1mol L -1 ~10 -7 mol L -1 The gradient solution;
[0033] Use a microsampler to pipette the organic solution of the fluorescent probe prepared in step I, and add it to the prepared gradient solution respectively, so that the final concentration of the fluorescent probe in the gradient solution is 5×10 -7 mol L -1 . Ultrasonic treatment was performed at 100...
Embodiment approach 2
[0048] Ⅰ Preparation of organic solution of fluorescent probe:
[0049] Using Nile Red as a fluorescent probe, dissolve Nile Red in methanol and prepare a concentration of 10 in a 10 mL volumetric flask. -1 mol L -1 Methanol solution of Nile red, further diluted with methanol to 10 -3 mol L -1 ;
[0050] Ⅱ Determination of critical micelle concentration CMC of surfactant:
[0051] Dissolve sodium tetradecyl sulfate in a phosphate buffer solution with a concentration of 10 mM and pH 8.0, and prepare 10 in a 10 mL volumetric flask -2 mol L -1 The solution is further diluted with buffer solution to obtain a concentration range of 1mol L -1 ~10 -7 mol L -1 The gradient solution;
[0052] Use a microsampler to pipette the organic solution of the fluorescent probe prepared in step I, and add it to the prepared gradient solution respectively, so that the final concentration of the fluorescent probe in the gradient solution is 10 -6 mol L -1 . Ultrasonic treatment was...
Embodiment approach 3
[0067] Ⅰ Preparation of organic solution of fluorescent probe:
[0068] Using Sudan Red as a fluorescent probe, dissolve Sudan Red in chloroform and prepare a concentration of 10 in a 10 mL volumetric flask. -1 mol L -1 Chloroform solution of Sudan Red, further diluted with chloroform to 10 -3 mol L -1 ;
[0069] Ⅱ Determination of critical micelle concentration CMC of surfactant:
[0070] Sodium cetyl sulfate was dissolved in a phosphate buffer solution with a concentration of 10 mM and pH 8.0, and 10 -2 mol L -1 The solution is further diluted with buffer solution to obtain a concentration range of 1mol L -1 ~10 -7 mol L -1 The gradient solution;
[0071] Use a microsampler to pipette the organic solution of the fluorescent probe prepared in step I, and add it to the prepared gradient solution respectively, so that the final concentration of the fluorescent probe in the gradient solution is 10 -6 mol L -1 . Ultrasonic treatment was performed at 40 kHz for 60 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com