Mobile phase for separating levodopa in broad beans in high efficiency liquid chromatography
A high-performance liquid chromatography and levodopa technology, which is applied in the mobile phase field of separating levodopa in broad beans in high-performance liquid chromatography, can solve problems such as seal ring friction, damage to the pump system, damage, etc., to reduce damage, The effect of increasing the pH value and facilitating quantitative analysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
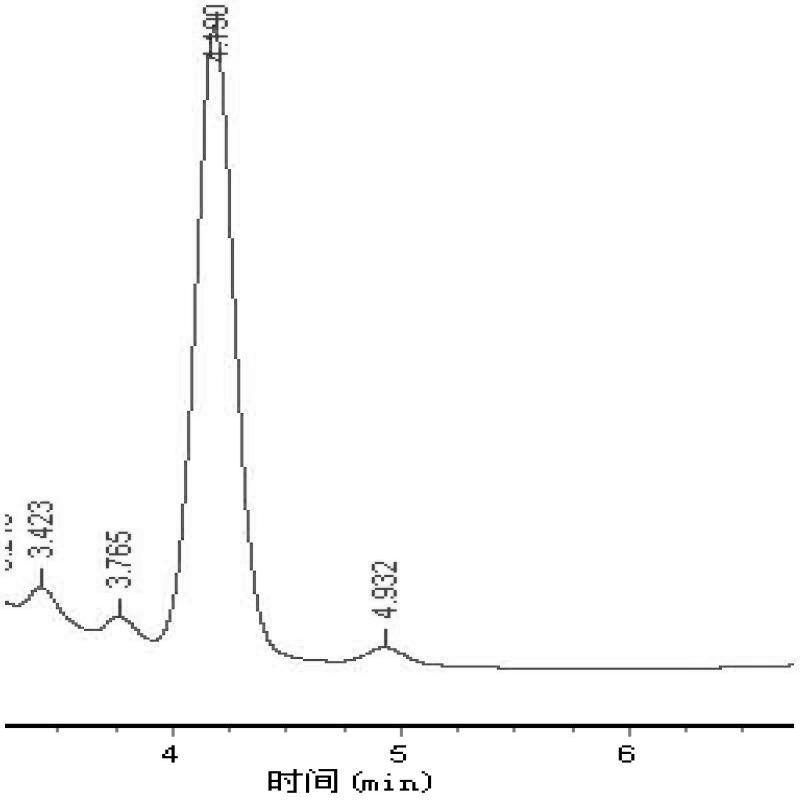
[0035] Example 1, such as figure 1 Or as shown in 2, the mobile phase is composed of 95% by volume of 0.1% formic acid and 5% acetonitrile, and the HPLC-UV chromatograms obtained by separating and detecting the levodopa standard and the levodopa extracted from Vicia faba flower respectively . Chromatographic column is Shiseido CAPCELL PAK CR column (150 mm×4.6 mm, 5 μm); mobile phase flow rate: 1.0 mL / min; detection wavelength: 280 nm; column temperature: 30 ℃; injection volume: 20 μL, in the chromatogram The abscissa is the retention time of the levodopa standard and the levodopa extracted from the broad bean flower passing through the chromatographic column, and the time is in minutes, and the ordinate is the levodopa standard and the levodopa extracted from the broad bean flower passing through the chromatographic column Response value voltage, the voltage response value is in volts (the following examples use the same chromatographic column conditions as this example). f...
Embodiment 2
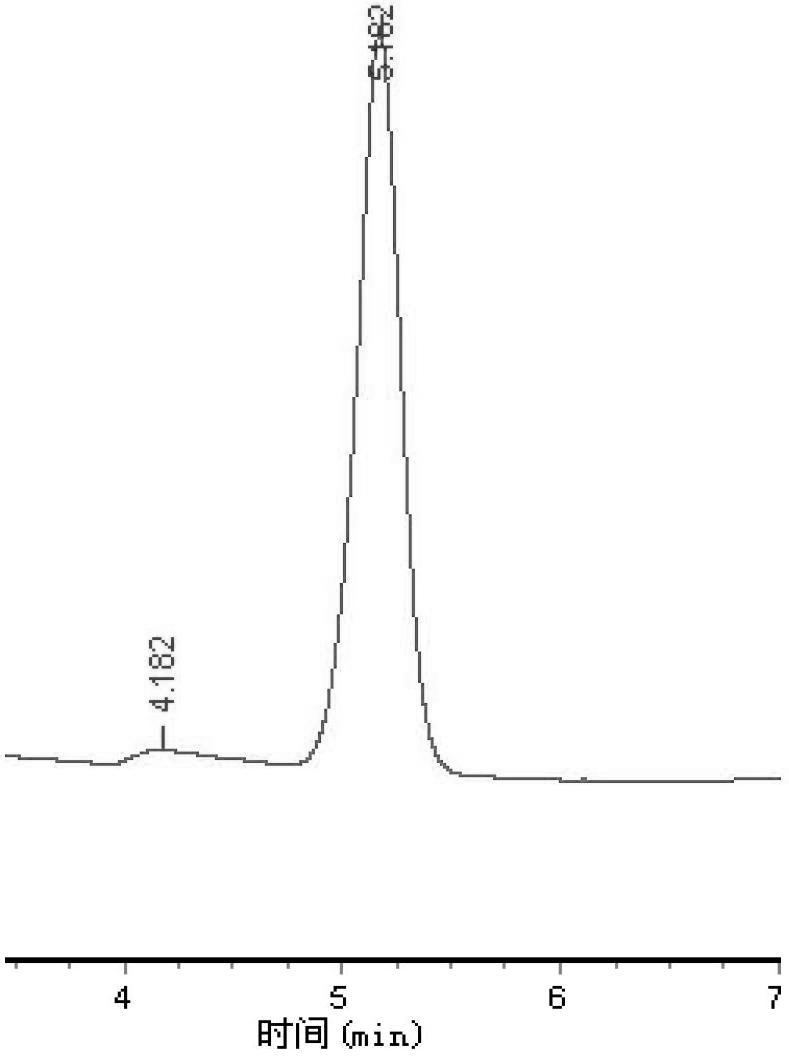
[0036] Example 2, such as image 3Or as shown in 4, adopt mobile phase composition volume percent to be: 0.1% formic acid 90%, acetonitrile 9%, 0.1 mol / L acetic acid 1%, the levodopa standard substance and the levodopa extracted in the broad bean flower are separated respectively Detect the resulting HPLC-UV chromatogram, figure 1 As shown in the chromatogram, the mobile phase can completely separate the baseline of levodopa, and the peak area of the peak 4.465 has a good linear correlation with the mass, but the baseline drift is obvious. figure 2 As shown in the chromatogram, the mobile phase can completely separate the baseline of levodopa and some unknown substances, and the peak area of the peak 4.490 has a good linear correlation with the mass, but the baseline drift is obvious.
Embodiment 3
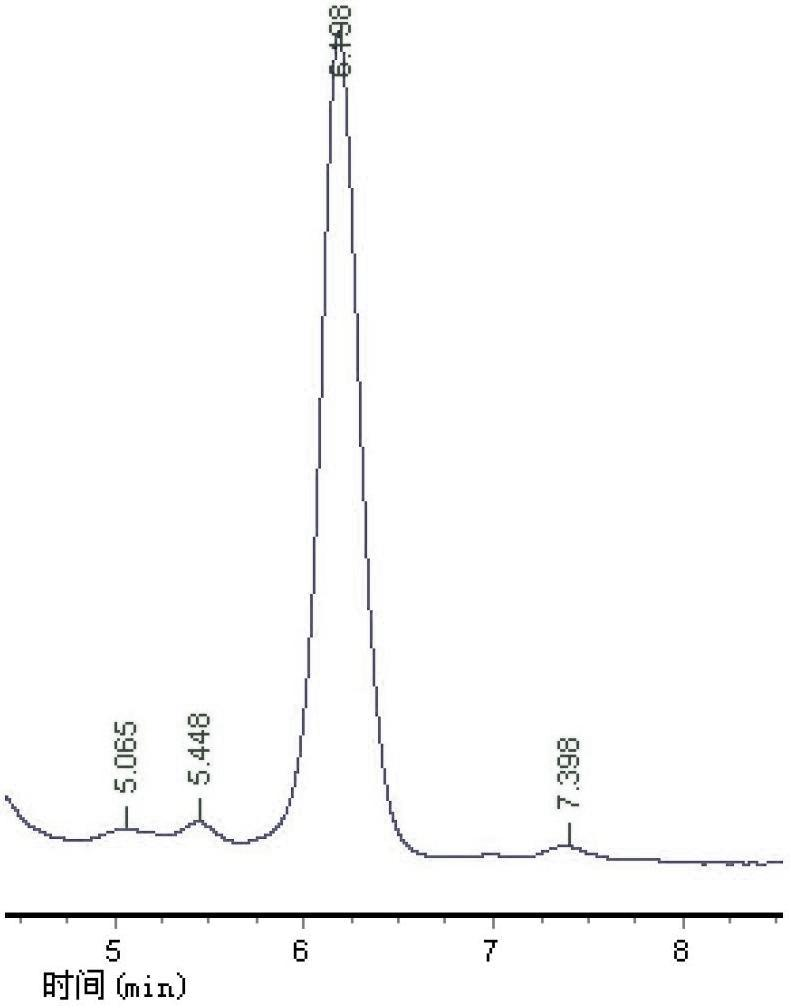
[0037] Example 3, such as Figure 5 Or as shown in 6, adopt mobile phase composition volume percentage to be: 0.1% formic acid 97%, acetonitrile 2%, 0.1 mol / L acetic acid 1%, the levodopa standard substance and the levodopa extracted in the broad bean flower are separated respectively According to the HPLC-UV chromatogram obtained by detection, the separation of levodopa between the standard levodopa and the faba bean flower sample is better in this mobile phase, and the retention time reaches more than 6 minutes, but the baseline drift of the two is obvious.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com