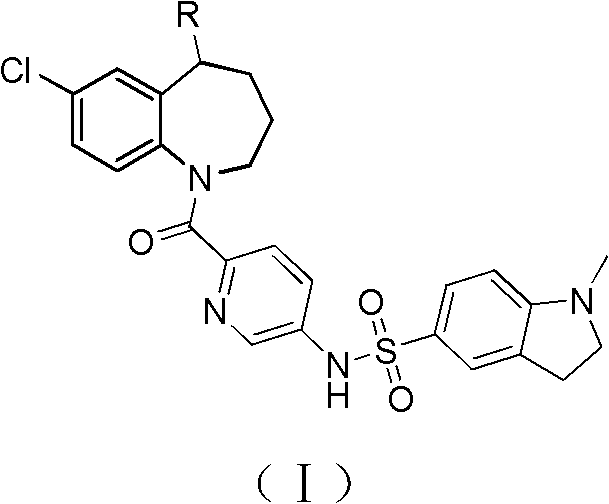
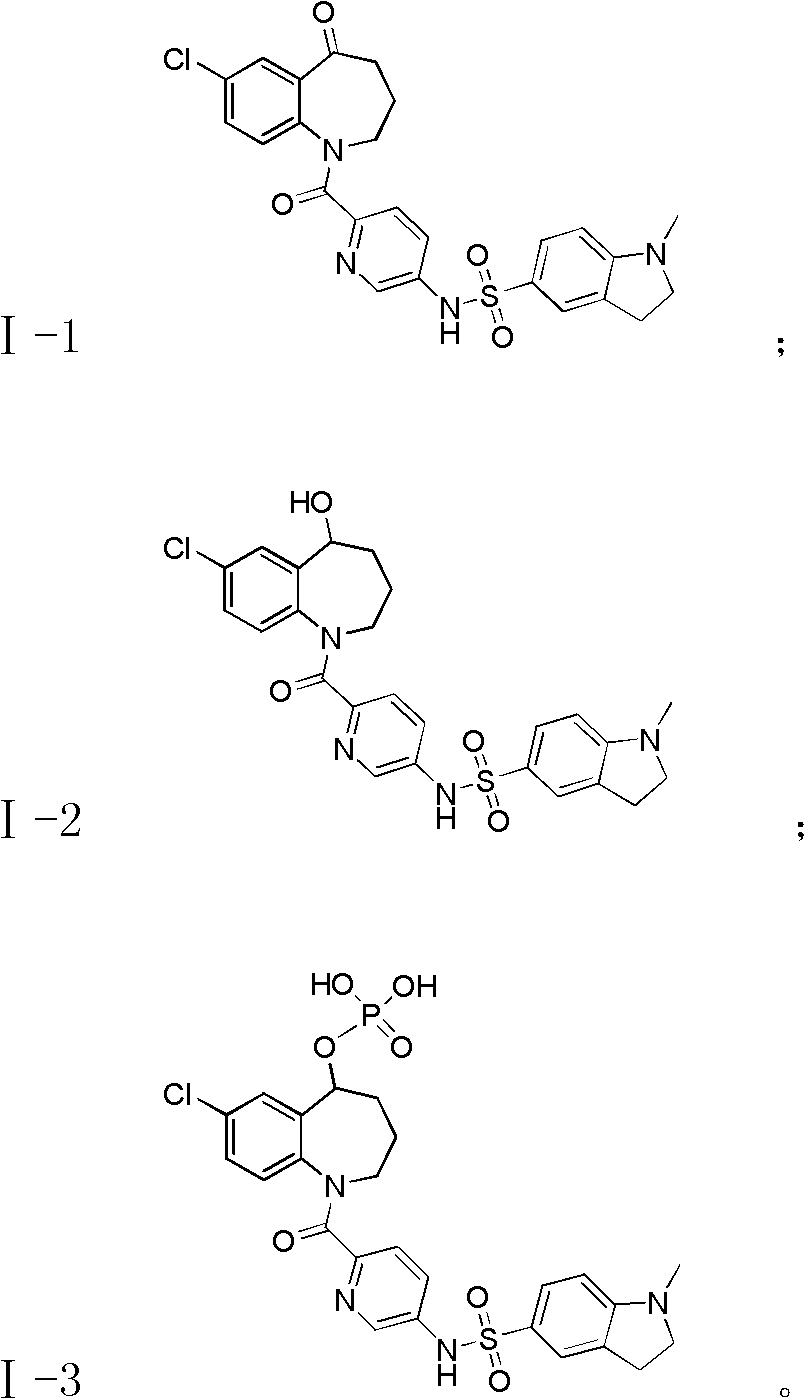
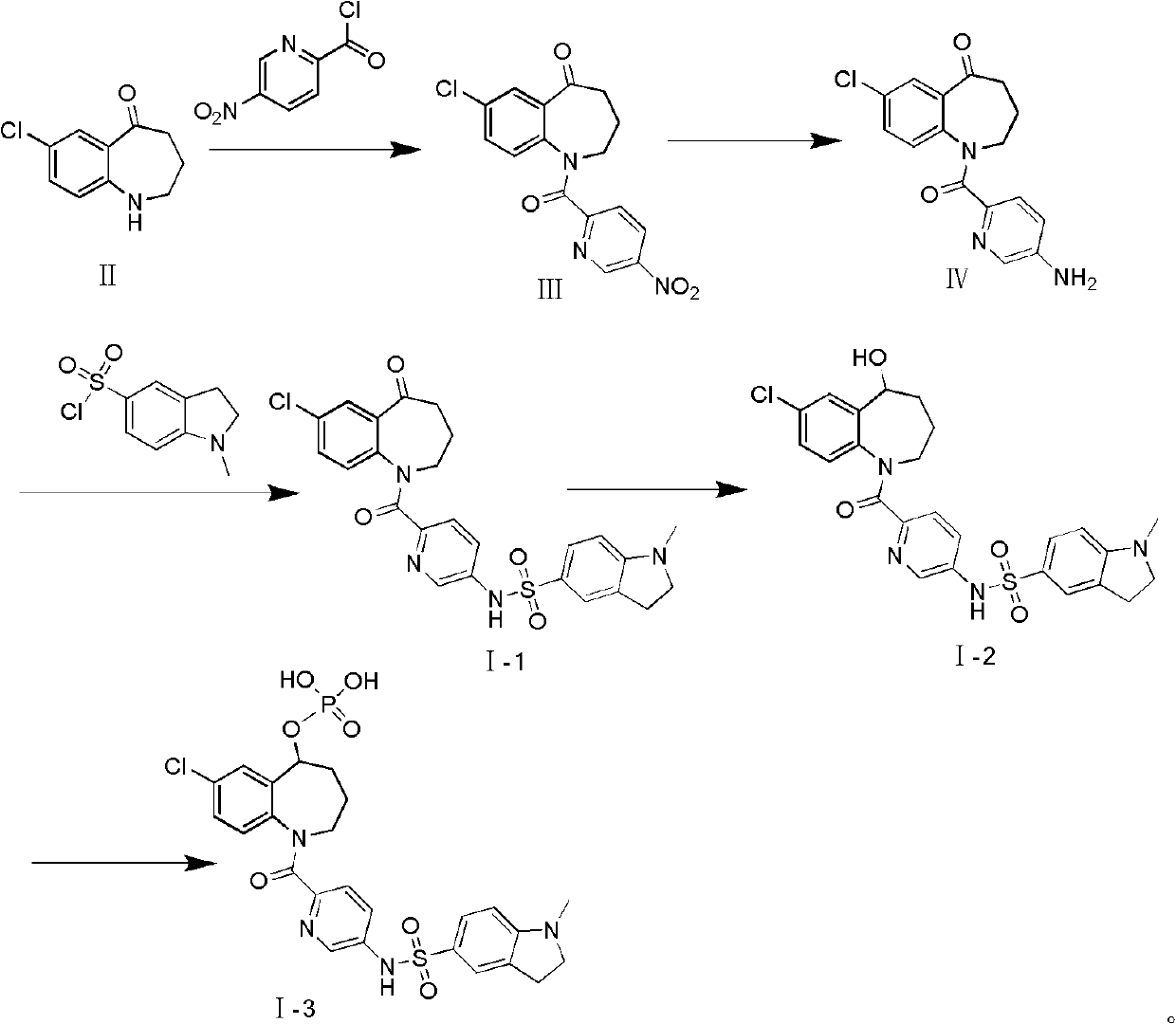
Compound with function of diuresis, preparation method and functions thereof
A compound and drug technology, applied in the field of medicine, can solve the problems of dosage form and route of administration, insufficient intestinal absorption, low water solubility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Preparation of Intermediate III
[0047]
[0048] Add II (50g, 260mmol), triethylamine (40g, 390mmol) and dichloromethane (350ml) to a 1000ml reaction flask, cool to 0°C in an ice bath, stir until it dissolves, and add 5-nitro-2 -Pyridine acid chloride (57.2g, 300mmol), react at room temperature for 30 minutes. TLC [developing agent: ethyl acetate-petroleum ether (1:1), the same below] After detecting that the reaction is complete, add 100 ml of a saturated solution of sodium bicarbonate to the reaction solution, stir for 10 minutes, filter, and filter the cake with dichloromethane (50ml×3)), combined the organic phases, washed with saturated brine (50ml×3), dried over anhydrous sodium sulfate, and filtered. The solvent was recovered from the filtrate under reduced pressure to obtain a crude product, which was recrystallized from absolute ethanol to obtain 55.1 g (62.4%, HPLC 95.5%) of a light yellow powder.
Embodiment 2
[0050] Preparation of Intermediate IV
[0051]
[0052] Add intermediate III (50g, 145mmol) to the 1000ml reaction flask, then add 250ml of dehydrated alcohol and 200ml of concentrated hydrochloric acid, stir for half an hour, slowly add dropwise 150ml of ethanol solution of stannous chloride (115g, 510mmol) in the mixed solution . React at 30°C for four hours. TLC [developer: ethyl acetate-petroleum ether (1:1)] detected that after the reaction was complete, distilled about 300 ml of ethanol under reduced pressure, cooled, precipitated solid, and filtered. Pour the filter cake into 500ml of water, adjust the pH to 9 with about 300ml of 20% sodium hydroxide solution, and filter to obtain a crude product. Recrystallized from absolute ethanol to obtain 31.6 g (69.2%, HPLC 96.6%) of light yellow solid powder.
Embodiment 3
[0054] Preparation of Compound I-1
[0055]
[0056] Add intermediate IV (30g, 95mmol) and pyridine (100ml) successively to a 250ml reaction flask equipped with stirring and a thermometer, stir to dissolve, and then add 1-methylindoline-5-sulfonic acid to the mixed solution dropwise. Pyridine solution of acid chloride (23g, 100mmol) was reacted at room temperature for 1 hour. After the reaction was complete as detected by TLC, it was poured into ice water, fully stirred, filtered, and the obtained solid was recrystallized through anhydrous methanol-petroleum ether (2:1) to obtain light Yellow solid powder 42.7g, (88%, HPLC 96.1%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com