Zinc phthalocyanine complex and preparation method thereof
A technology of zinc phthalocyanine and complexes, which is applied in the field of organic and metal coordination compound synthesis, can solve the problems of many side reactions, difficult synthesis, difficult separation, etc., and achieves the advantages of less side reactions, easily available raw materials, and simple synthesis method. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
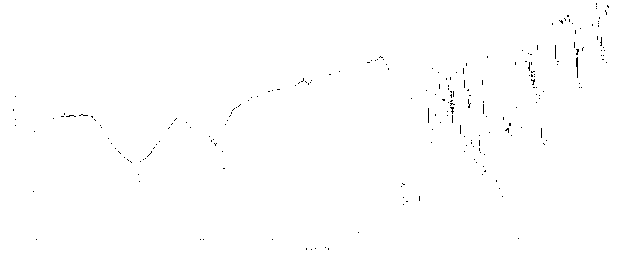
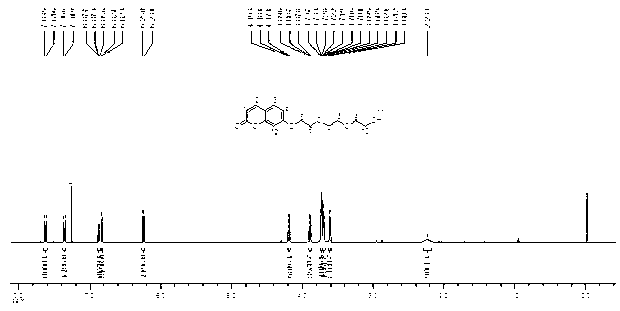
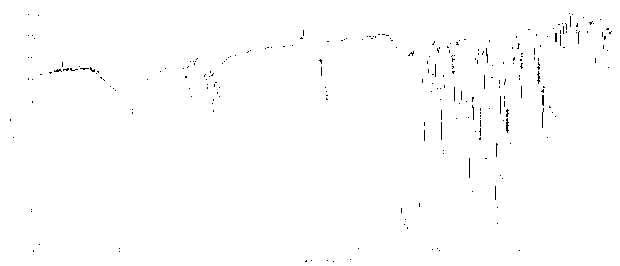
Image
Examples
Embodiment 1
[0074] 1) Synthesis of 8-hydroxy-3,6-dioxahoctyl p-toluenesulfonate: 32.00g (0.30mol) triethylamine, 24.00g (0.16mol) triethylene glycol and 7.60g (0.04mol) p-toluenesulfonate Benzenesulfonyl chloride was reacted in dichloromethane (70ml) system at 25°C for 10 hours. After the reaction was completed, it was extracted with 200ml of 1M hydrochloric acid solution, saturated saline, and distilled water. The crude product was separated by silica gel column chromatography using petroleum ether-ethyl acetate (1:1, v / v) as the eluent to obtain 5.48 g (0.018 mol) of the product with a yield of 45%.
[0075] 2) Synthesis of 8-(7-coumarinyloxy)-3,6-dioxa-1-octanol: 2.76g (20mmol) anhydrous potassium carbonate, 1.52g (5mmol) 8-hydroxy-3,6 -Dioxa-octyl p-toluenesulfonate and 0.81 g (5 mmol) 7-hydroxycoumarin were reacted in N,N-dimethylformamide (DMF, 15ml) at 60°C for 8 hours, and the reaction After the end, the solvent was evaporated, and the crude product was extracted with dichlorome...
Embodiment 2
[0079] The preparation method is the same as Example 1. In the process of (1), the molar ratio of triethylamine, triethylene glycol and p-toluenesulfonyl chloride is 1:1:1, and react at 0°C for 12 hours in a dichloromethane system , other operations were the same, and the yield of 8-hydroxyl-3,6-dioxahoctyl p-toluenesulfonate was 38%. In the process of (2), the molar ratio of anhydrous potassium carbonate, 8-hydroxy-3,6-dioxahoctyl p-toluenesulfonate and 7-hydroxycoumarin is 3:1:1.2, in DMF at The reaction was carried out at 25°C for 12 hours. After the reaction was completed, the solvent was evaporated, and the crude product was extracted with chloroform, followed by methanol-chloroform as the eluent, and separated by silica gel column chromatography. The yield was 87%. In the process of (3), the molar ratio of anhydrous potassium carbonate, 8-(7-coumarinoxy)-3,6-dioxa-1-octanol and 3 or 4-nitrophthalonitrile The ratio is 5:1:1.2, reacted in DMF at 60°C for 12 hours, other o...
Embodiment 3
[0081] The preparation method is the same as Example 1. In the process of (1), the molar ratio of triethylamine, triethylene glycol and p-toluenesulfonyl chloride is 2:2:1, and react at 0°C for 12 hours in a dichloromethane system , other operations were the same, and the yield of 8-hydroxyl-3,6-dioxahoctyl p-toluenesulfonate was 41%. In the process of (2), the molar ratio of anhydrous potassium carbonate, 8-hydroxy-3,6-dioxahoctyl p-toluenesulfonate and 7-hydroxycoumarin is 3:1:1.5, in DMF at Reacted at 60°C for 24 hours. After the reaction, the solvent was evaporated, and the crude product was extracted with chloroform, followed by methanol-chloroform as eluent, and separated by silica gel column chromatography. The yield was 62%. In the process of (3), the molar ratio of anhydrous potassium carbonate, 8-(7-coumarinoxy)-3,6-dioxa-1-octanol and 3 or 4-nitrophthalonitrile The ratio is 4:1:1.5, reacted in DMF at 60°C for 12 hours, other operations are the same as in Example 1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com