Recombinant protein of mycobacterium tuberculosis Rv 3120, preparation method and application in cellular immunological diagnosis thereof
A technology of Mycobacterium tuberculosis, rv3120, applied in the application of cellular immune diagnosis, the field of recombinant protein of the new tuberculosis immune marker Rv3120, can solve the problems of low diagnostic value and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Construction of embodiment 1 recombinant plasmid pET32a-Rv3120
[0059] (1) Target gene primer design
[0060] Rv3120-F (SEQ ID No: 3): CAA GGTACC ATGAGTCCGTCTCCATCG
[0061] Rv3120-R (SEQ ID No: 4): CCT AAGCTT CTACAGTGACCGTTGGGC
[0062] The enzyme cutting sites are Kpn I and Hind III respectively.
[0063] (2) PCR amplification, cloning and sequence determination of the target gene
[0064] Using Mycobacterium tuberculosis H37Rv genomic DNA as a template, Rv3120-F and Rv3120-R as primers, the Rv3120 protein gene was directly amplified by PCR using Taq enzyme (Bao Bioengineering (Dalian) Co., Ltd.). PCR reaction conditions: pre-denaturation at 94°C for 5 minutes; (94°C, 30s; 58°C, 30s; 72°C, 40s) 35 cycles; extension at 72°C for 5 minutes; storage at 4°C. After the reaction, the target fragment was separated by 1% agarose gel electrophoresis, and then recovered with a DNA recovery kit (Invitrogen). Digested with Kpn I and Hind III, and cloned into pET32a plasmi...
Embodiment 2
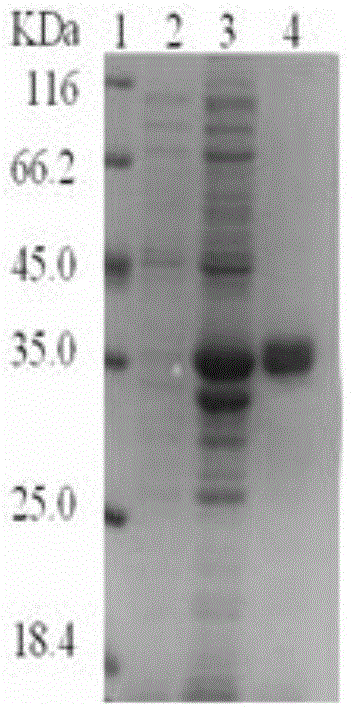
[0065] Example 2 Induced expression and purification of recombinant protein Rv3120
[0066] Put 0.5ul of the recombinant pET32a-Rv3120 plasmid with correct sequencing into 100ul of Escherichia coli BL21 (DE3) physS competent cells (TIANGEN), place it on ice for 45min, put it in a water bath at 42°C, heat shock for 90s, and let it stand on ice for 3min. Add 500ul of preheated LB medium without antibiotics, shake at 37°C, 220rmp, and incubate for 45-60min. Take a certain amount and spread it on a solid LB medium plate containing kanamycin, dry it at room temperature and place it upside down in a 37°C incubator for overnight cultivation. Pick clones, put them into LB liquid medium containing 50ug / ml kanamycin resistance, culture at 220rmp, 37°C to OD to about 0.6, add final concentration of 10mM IPTG, 37°C for 3h, collect IPTG-induced Bacteria were resuspended according to the ratio of 10ml Washing Buffer I per gram of wet bacteria, and then ultrasonically disrupted. Results Th...
Embodiment 3
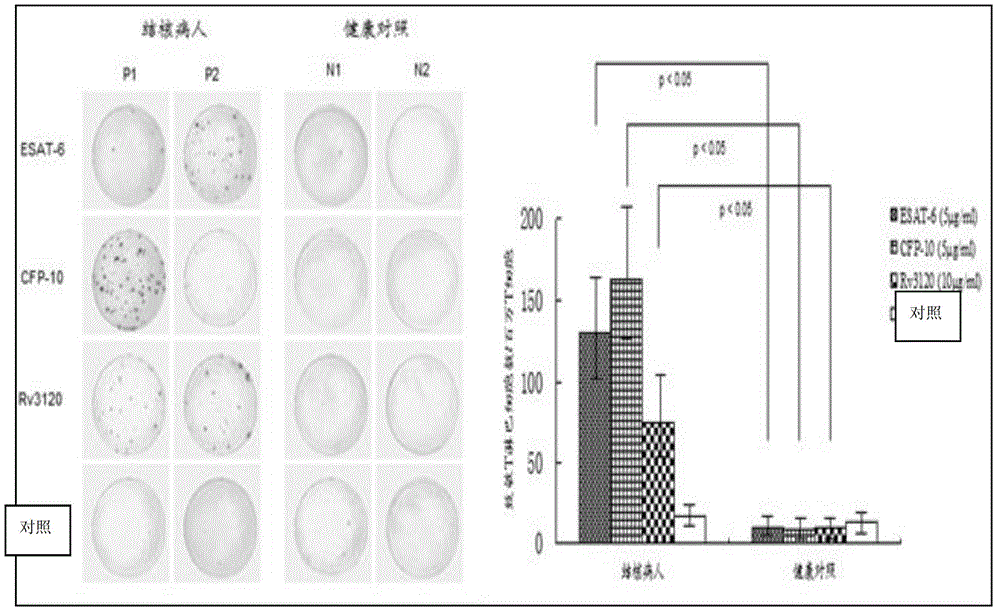
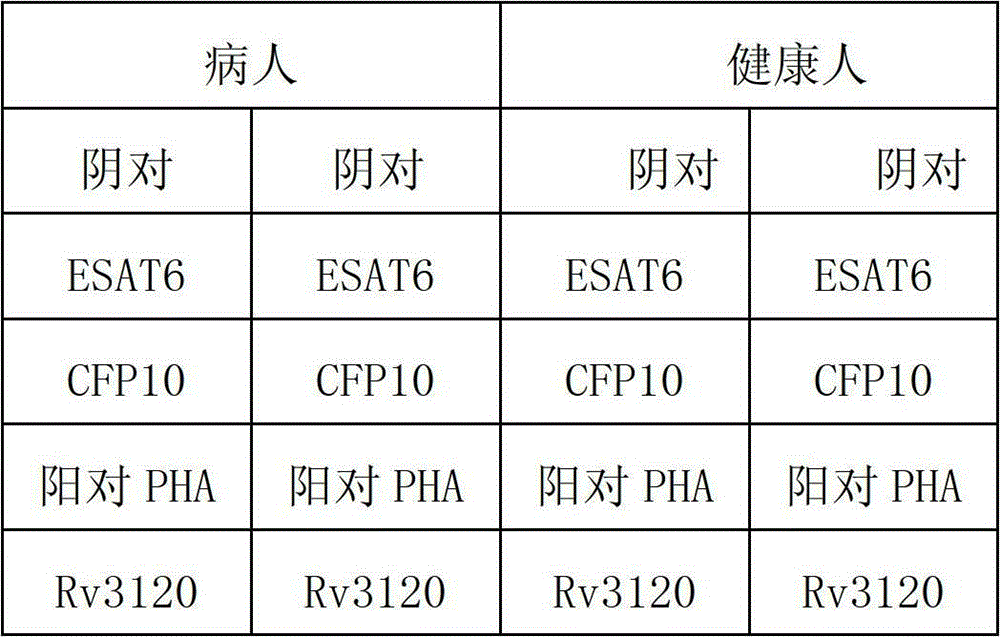
[0068] Example 3 Recombinant Rv3120 antigen is used as a detection reagent to detect clinically suspected tuberculosis patients.
[0069] The specific steps are:
[0070] Steps:
[0071] 1. Sample collection: Aseptically collect about 5ml of human peripheral venous blood into a heparin anticoagulant tube. After collection, the sample can be stored at room temperature and not placed in a refrigerator or freezer; and marked.
[0072] 2. Separation, collection and counting of peripheral blood mononuclear lymphocytes:
[0073] a. Take 5ml of whole blood, add an equal volume of RTPMI-1640 serum-free culture medium, and mix well.
[0074] b. Take a 15ml centrifuge tube and add 4ml of lymphocyte separation medium, and slowly add the mixed blood sample to the surface of the lymphocyte separation medium. , handle with care.
[0075] c. Put the centrifuge tube into a horizontal centrifuge at 1000g, room temperature, and centrifuge for 22 minutes. After careful removal, the blood co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com