High fluorescence emitted POSS substituted perylene diimide crystal and preparation method
A perylene diimide and fluorescence emission technology, applied in chemical instruments and methods, luminescent materials, silicon organic compounds, etc., can solve the problem of low fluorescence quantum yield of solid perylene imide derivatives and achieve high stability , strong absorption, and enhanced fluorescence quantum yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051]
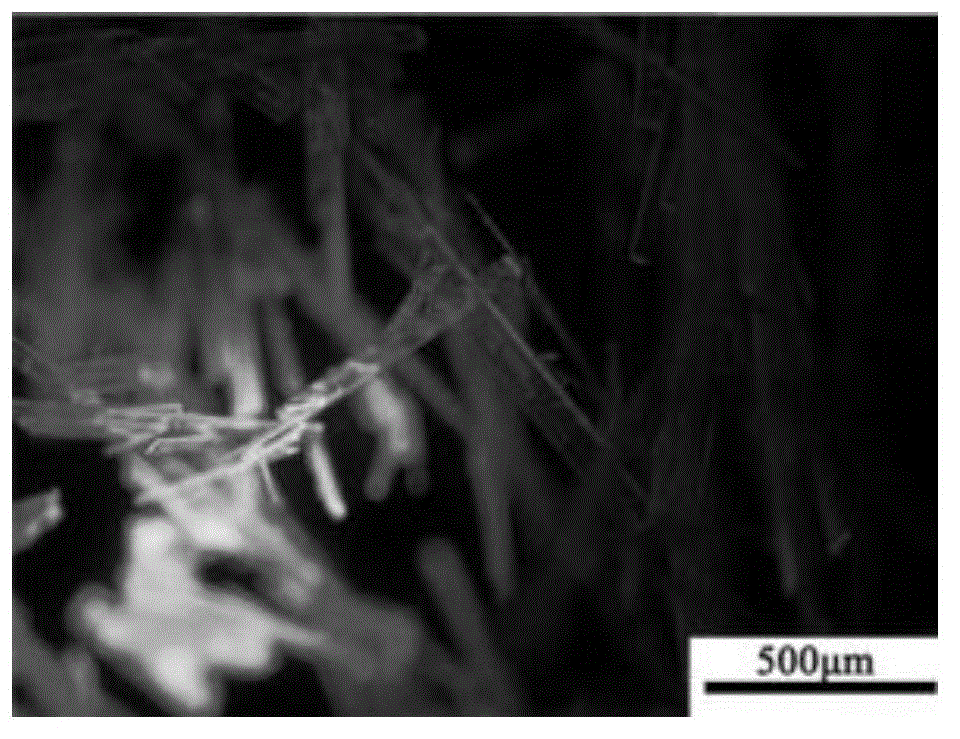
[0052] Preparation of compound crystals of formula (III): use good solvent chloroform to configure unsaturated solution (7.4 mg / ml) of compound of formula (III); configure the crystal growth system in the container from bottom to top as the above-mentioned unsaturated solution, The buffer layer (volume ratio 1:1) of the good solvent chloroform / poor solvent methanol mixture and the poor solvent methanol layer, the unsaturated solution layer, the buffer layer, and the volume ratio of the methanol layer are 3:5:30; with the solvent Interdiffusion between layers, precipitation at the bottom of the container as image 3 Flat ribbon-shaped crystals of the compound of formula (III) shown.
[0053] see figure 1 , the crystal fluorescence emission peak (11) of the compound of formula (III) is 624 nm, and the fluorescence emission peak (12) of the compound of formula (III) is 649 nm in the amorphous solid.
[0054] see figure 2 , the characteristic peaks of the X-ray dif...
Embodiment 2
[0057]
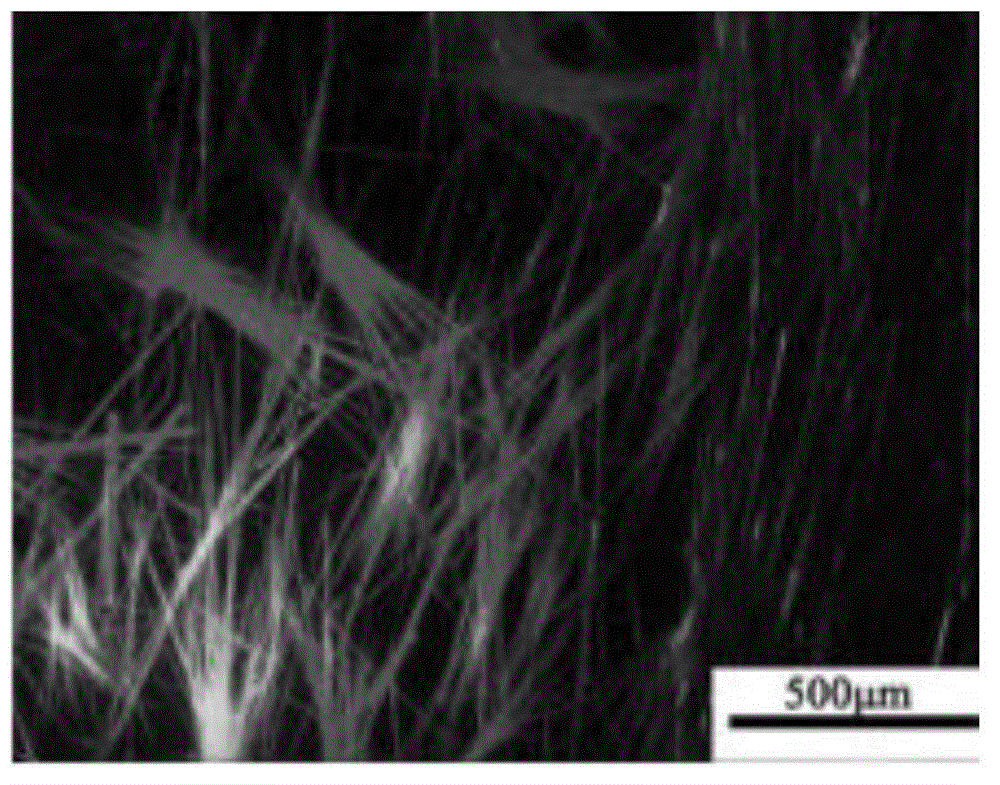
[0058] Preparation of compound crystals of formula (IV): Add 1 mg of amorphous solid of formula (IV) to 5 ml of chloroform solvent, add the resulting solution dropwise into acetone, a poor solvent, and immediately precipitate as Image 6 Needle crystals of the compound of formula (IV);
[0059] see Figure 4 , the fluorescence emission peak (21) of the formula (IV) compound crystal is 646 nm, and the fluorescence emission peak (22) of the formula (IV) compound amorphous solid is 658 nm.
[0060] see Figure 5 , the characteristic peaks of the X-ray diffraction pattern (23) of the compound of formula (IV) show that the compound of formula (IV) is amorphous no matter in a small angle with a 2θ angle of less than 10° or in a wide angle between 10° and 60°. The solid diffraction pattern (24) has many sharp diffraction peaks, showing obvious crystal features.
[0061] The fluorescence quantum yield of the crystal of the compound of formula (IV) is 35.57%, and the flu...
Embodiment 3
[0063]
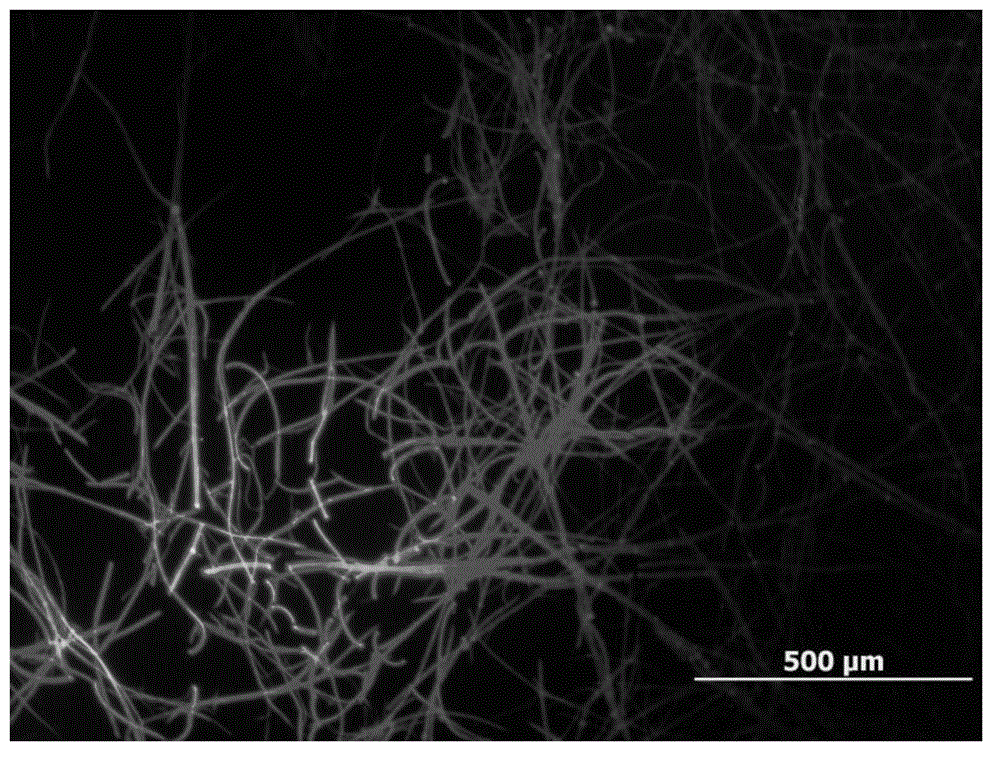
[0064] Preparation of compound crystals of formula (V): Add THF as a good solvent into a petri dish, cover with a watch glass and place it for 30-60 minutes to form a saturated atmosphere of THF; place the mica sheet in a saturated atmosphere of THF, and then place the ) compound tetrahydrofuran dilute solution (0.5mg / ml) was dropped on the mica sheet, quickly cover the petri dish, and put the whole device in a non-disturbing environment to prevent the crystal growth process from being affected. After the solvent has evaporated, it will form on the mica sheet such as Figure 9 Ribbon crystals of the compound of formula (V) shown.
[0065] Such as Figure 7 , the fluorescence emission peak (31) of the formula (V) compound crystal is 622nm, and the fluorescence emission peak (32) of the formula (V) compound amorphous solid is 648nm.
[0066] Such as Figure 8 , the characteristic peaks of the X-ray diffraction pattern (33) of the compound of formula (V) are higher...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com