Polyaryletherketone anion conducting membrane material with side chain containing quaternary ammonium salt group and its preparation method
A technology of quaternary ammonium salt group and polyaryletherketone, which is applied in the direction of electrical components, circuits, battery electrodes, etc., can solve the problem of membrane mechanical performance degradation and achieve excellent thermal performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Preparation of side chain-containing methylphenyl polyaryletherketone (X=0.6) (1)
[0032] 0.6428 grams (0.003mol) of 3,5-dimethylphenylhydroquinone, 0.6725 grams (0.002mol) of 2,2-bis-(4-hydroxyphenyl)hexafluoropropane, 4,4'-bis Put 1.091 grams (0.005 mol) of fluorobenzophenone and 0.7602 grams of anhydrous potassium carbonate, 7 mL of N-methylpyrrolidone, and 7 mL of toluene into a 50 mL three-necked flask equipped with a water dispenser, ventilate argon, and heat up to the reflux of toluene. Stir and reflux for 2 hours, remove toluene, raise the temperature to 180°C, continue to react at about 180°C for 6 hours, precipitate the polymer solution in deionized water, grind, wash, and dry to obtain polyarylene with side groups containing methylphenyl White powder of ether ketone. The molecular formula is as follows:
[0033]
Embodiment 2
[0034] Embodiment 2: the preparation (2) of polyaryletherketone containing methylphenyl in side chain
[0035] The method is the same as in Example 1, and the amount of monomer is enlarged tenfold to carry out polymerization. 6.428 grams (0.03mol) of 3,5-dimethylphenylhydroquinone, 6.725 grams (0.02mol) of 2,2-bis-(4-hydroxyphenyl)hexafluoropropane, 4,4'-difluoro 10.910 grams of benzophenone (0.05mol) and 7.602 grams of anhydrous potassium carbonate, 70mL of N-methylpyrrolidone, and 50mL of toluene are put into a 250mL three-necked flask equipped with a water dispenser, argon gas is passed, the temperature is raised to toluene reflux, and the mixture is stirred. Reflux for 3 hours, remove toluene, heat up to 180°C, continue to react at about 180°C for 12 hours, precipitate the polymer solution in deionized water, grind, wash, and dry to obtain the same side group-containing methyl group as in Example 1. White powder of phenyl polyaryletherketone.
Embodiment 3
[0036] Example 3: Preparation of side chain-containing methylphenyl polyaryletherketone (X=0.75) (1)
[0037] 0.8034 g (0.00375 mol) of 3,5-dimethylphenylhydroquinone, 0.4203 g (0.00125 mol) of 2,2-bis-(4-hydroxyphenyl) hexafluoropropane, 4,4'-di Put 1.091 grams (0.005 mol) of fluorobenzophenone and 0.7602 grams of anhydrous potassium carbonate, 7 mL of N-methylpyrrolidone, and 7 mL of toluene into a 50 mL three-necked flask equipped with a water dispenser, ventilate argon, and heat up to the reflux of toluene. Stir and reflux for 2 hours, remove toluene, raise the temperature to 180°C, continue to react at about 180°C for 6 hours, precipitate the polymer solution in deionized water, grind, wash, and dry to obtain polyarylene with side groups containing methylphenyl White powder of ether ketone. The molecular formula is as follows:
[0038]
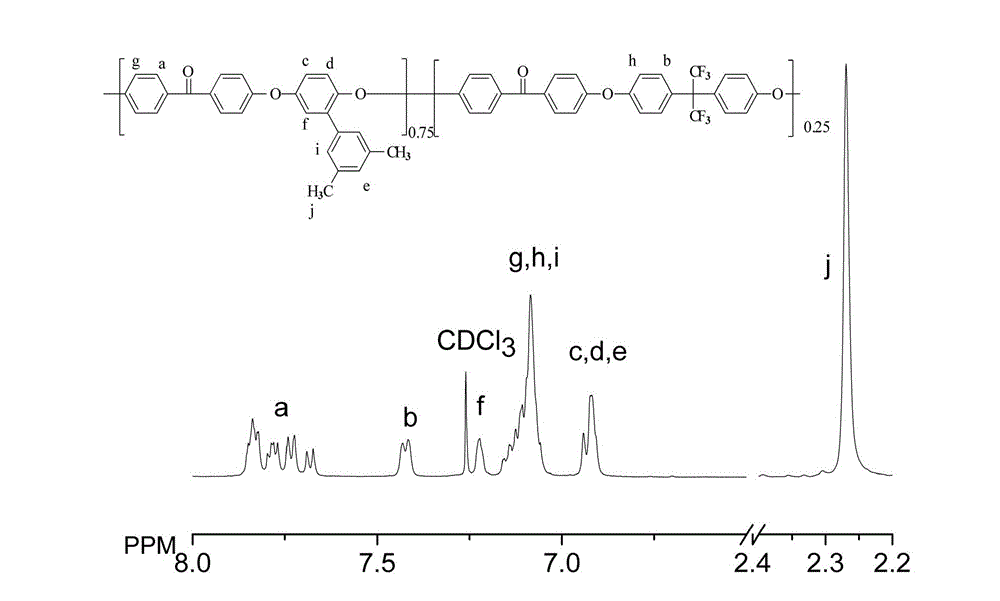
[0039] See figure 1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com