Sustained release preparation containing ambroxol hydrochloride and clenbuterol hydrochloride, and preparation method thereof
A technology of clenbuterol hydrochloride and ambroxol hydrochloride, which is applied in the preparation field of the sustained-release preparation, can solve the problems of easily causing adverse reactions, fluctuation of blood drug concentration, poor patient compliance, etc. The effect of stable drug concentration and symptom relief
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
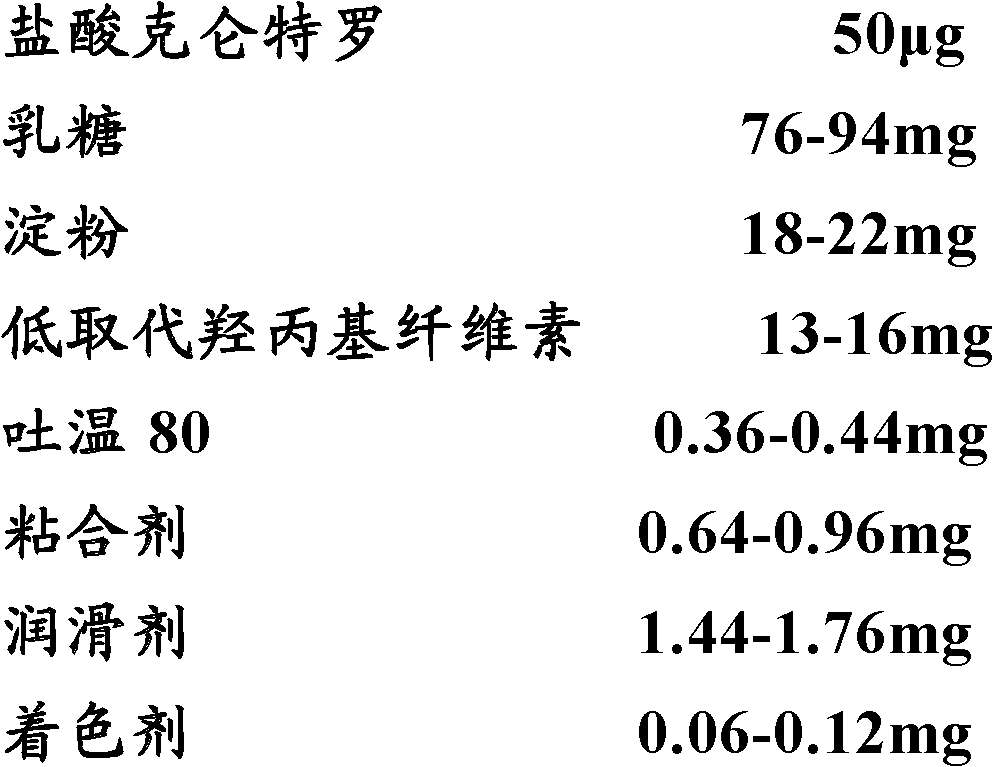
[0040] Example 1: Low specification compound ambroterol double-layer sustained-release tablet
[0041] prescription:
[0042]
[0043] Preparation:
[0044] Immediate-release layer: Weigh clenbuterol hydrochloride bulk drug, lactose, starch, and red iron oxide in the prescribed amount through an 80-mesh sieve, mix well, add Tween 80, stir well, and use an appropriate amount of 5% PVP aqueous solution as a viscous Mixture soft material, the dosage is subject to the actual dosage required for granulation, pass through a 20-mesh sieve to granulate, dry in an oven at 50°C for 1 hour, granulate with a 18-mesh sieve, and then add cross-linked carboxymethyl fiber Su Na, micronized silica gel and magnesium stearate, mix well.
[0045] Sustained-release layer: Weigh the ambroxol hydrochloride bulk drug, lactose, hypromellose (HPMC) K100LV and HPMC K4M that have passed the 80-mesh sieve, mix them evenly, and add an appropriate amount of 5% PVP ethanol solution as a binder For maki...
Embodiment 2
[0047] Example 2: Compound Ambroterol Double-Layer Sustained Release Tablets of Medium Specification
[0048] prescription:
[0049]
[0050] Preparation:
[0051] Immediate-release layer: Weigh the clenbuterol hydrochloride bulk drug, lactose, microcrystalline cellulose, and red iron oxide with the prescribed amount passed through an 80-mesh sieve, mix well, add Tween 80, stir well, and add an appropriate amount of 3% HPMC aqueous solution As a soft material made of binder, the dosage is subject to the actual dosage required for granulation, granulated with a 20-mesh sieve, dried in an oven at 50°C for 1 hour, granulated with a 18-mesh sieve, and then added with crospovidone , micronized silica gel and magnesium stearate, and mix well.
[0052] Sustained-release layer: Weigh the ambroxol hydrochloride raw material drug, lactose, carbomer and glyceryl behenate that have passed the 80-mesh sieve, mix them evenly, and add an appropriate amount of 5% PVP ethanol solution as a ...
Embodiment 3
[0054] Example 3: High specification compound ambroterol double-layer sustained-release tablet
[0055] prescription:
[0056]
[0057] Note: 10% starch slurry is the binder in this product, and its dosage is based on the amount required for actual granulation.
[0058] Preparation:
[0059] Immediate-release layer: Weigh the clenbuterol hydrochloride raw material, lactose, starch, and red iron oxide with the prescribed amount passed through an 80-mesh sieve, mix well, add Tween 80, stir well, and make a viscous layer with an appropriate amount of 10% starch slurry Mixture soft material, its dosage is subject to the actual dosage required for granulation, granulated with a 20-mesh sieve, dried in an oven at 50°C for 1 hour, granulated with a 18-mesh sieve, and then added with low-substituted hydroxypropyl cellulose, Micronized silica gel and magnesium stearate, mixed evenly.
[0060] Sustained-release layer: Weigh the ambroxol hydrochloride crude drug, lactose, acrylic r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com