Period effect percutaneous patch of self viscosity elastic body substrate containing testosterone and preparation method thereof
An elastomer and self-adhesive technology, applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, organic active ingredients, etc., can solve problems such as poor compliance, increased cost, and large drug loading. Achieve the effects of avoiding aging and oxidation, reducing heating time, and good adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
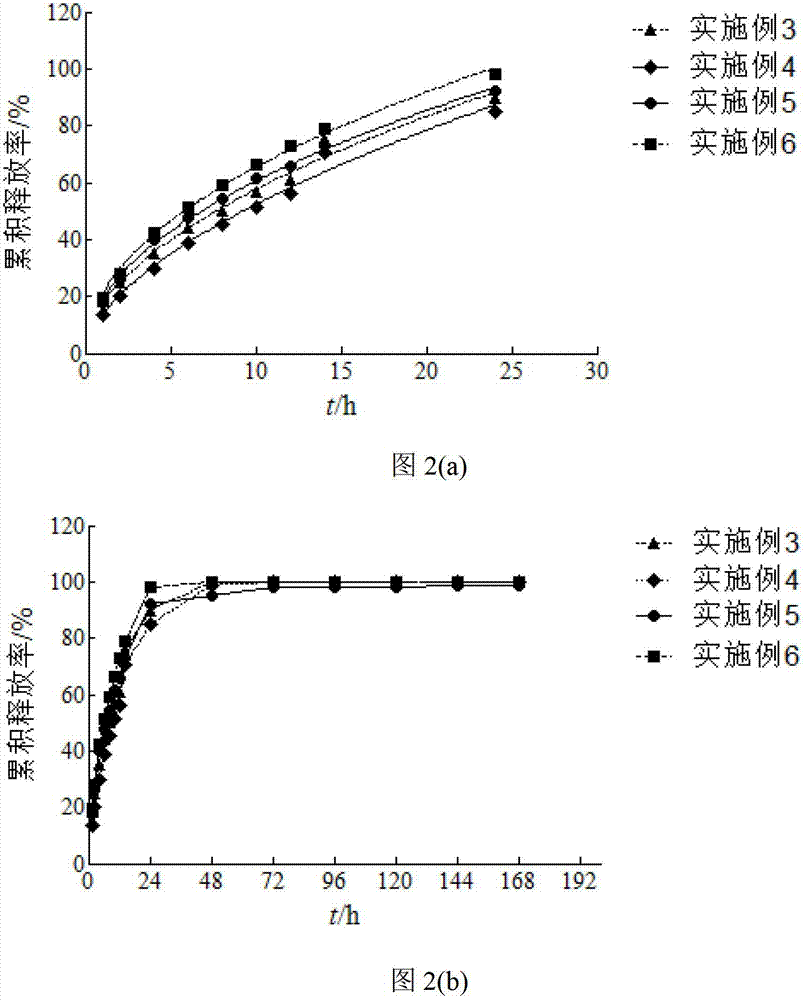
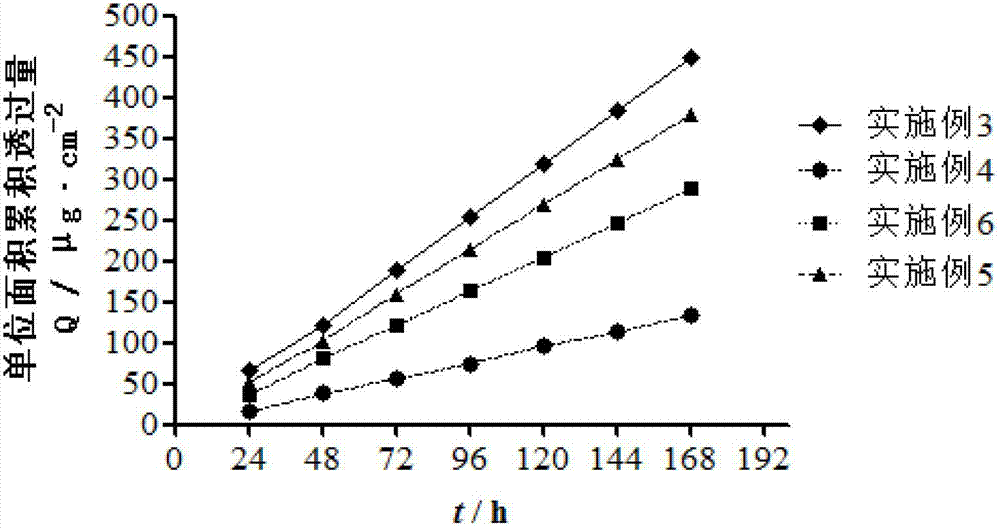
Examples
Embodiment 1
[0055] Preparation of lipophilic elastomer matrix with self-adhesive properties:
[0056] Take 100g of styrene-isoprene-styrene triblock copolymer (SIS), 140g of hydrogenated petroleum resin, 80g of naphthenic oil, 10g of isopropyl myristate, 5g of polyethylene glycol, N-methyl Pyrrolidone 15g, polyvinylpyrrolidone 10g, zinc oxide 10g, dibutylhydroxytoluene 2g. Heat SIS, naphthenic oil, isopropyl myristate, polyethylene glycol, N-methylpyrrolidone and dibutylhydroxytoluene to 160°C under nitrogen protection, and adjust the temperature to 100°C~ 120°C, add hydrogenated petroleum resin, polyvinylpyrrolidone and zinc oxide, continue heating and stirring for 20 minutes until a uniform and transparent colloid is formed, pour it out while it is hot, and cool it down.
[0057] It was determined that the melting point of the prepared lipophilic elastomer matrix with self-adhesive properties was 87°C.
Embodiment 2
[0059] Preparation of lipophilic elastomer matrix with self-adhesive properties:
[0060] Take 100g of styrene-isoprene-styrene triblock copolymer (SIS), 60g each of hydrogenated petroleum resin and hydrogenated rosin resin, 50g each of liquid paraffin and polybutene, 20g of diethyl phthalate, Polyethylene glycol 10g, lauryl lactate 10g, 2-ethylhexyl acrylate 20g, zinc oxide 10g, dibutylhydroxytoluene 5g. Heat SIS, liquid paraffin, polybutene, diethyl phthalate, polyethylene glycol, lauryl lactate and dibutyl hydroxytoluene to 180°C under nitrogen protection, and adjust the temperature after melting the components To 100℃~120℃, add hydrogenated petroleum resin, hydrogenated rosin resin, 2-ethylhexyl acrylate and zinc oxide, continue heating and stirring for 20 minutes until a uniform transparent colloid is formed, pour it out while it is hot, and cool it down.
[0061] It was determined that the melting point of the prepared lipophilic elastomer matrix with self-adhesive prop...
Embodiment 3
[0063] Preparation of a weekly transdermal composition containing testosterone:
[0064] [Prescription] (based on 1000 tablets)
[0065] Take 100g of styrene-isoprene-styrene triblock copolymer (SIS), 80g of hydrogenated petroleum resin, 40g of hydrogenated rosin resin, 60g of liquid paraffin, 40g of naphthenic oil, 10g of isopropyl myristate, poly 10g of ethylene glycol, 10g of N-methylpyrrolidone, 10g of methyl methacrylate, 5g of polyvinylpyrrolidone, 5g of zinc oxide, and 2g of dibutylhydroxytoluene.
[0066] Heat SIS, liquid paraffin, naphthenic oil, isopropyl myristate, polyethylene glycol, N-methylpyrrolidone and dibutyl hydroxytoluene to 160°C-180°C under nitrogen protection, after melting the components Adjust the temperature to 100°C-120°C, add hydrogenated petroleum resin, hydrogenated rosin resin, polyvinylpyrrolidone, methyl methacrylate and zinc oxide, continue heating under nitrogen protection until all components are stirred evenly; adjust the temperature to 7...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com