Quality control method for ribonucleic acid II for injection
A technology for ribonucleic acid and injection, applied in the field of detection of ribonucleic acid for injection II, which can solve the problems of low specificity and achieve the effects of improving technological content, reducing costs, and expanding production scale
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1, the optimization of high performance liquid chromatography detection ribonucleic acid II conditions for injection
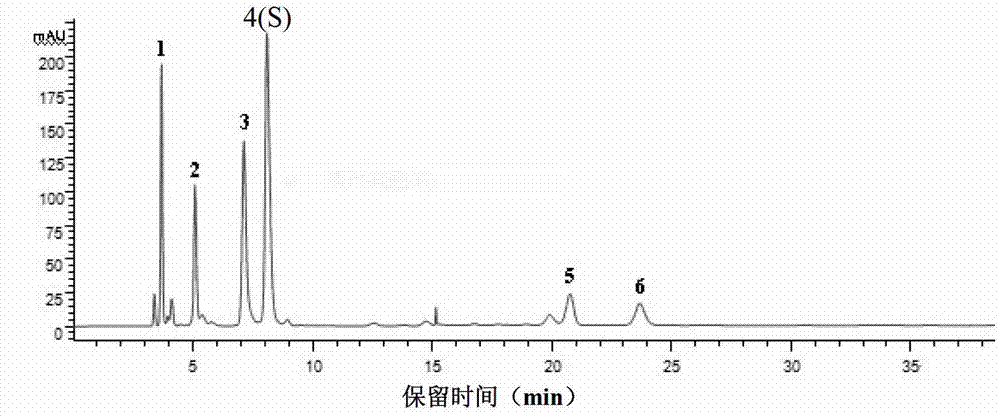
[0053] 1. Confirmation of common peaks in the characteristic spectrum of ribonucleic acid for injection II and identification of common chromatographic peaks
[0054] 1. Liquid chromatography
[0055] (1) Preparation of the test solution
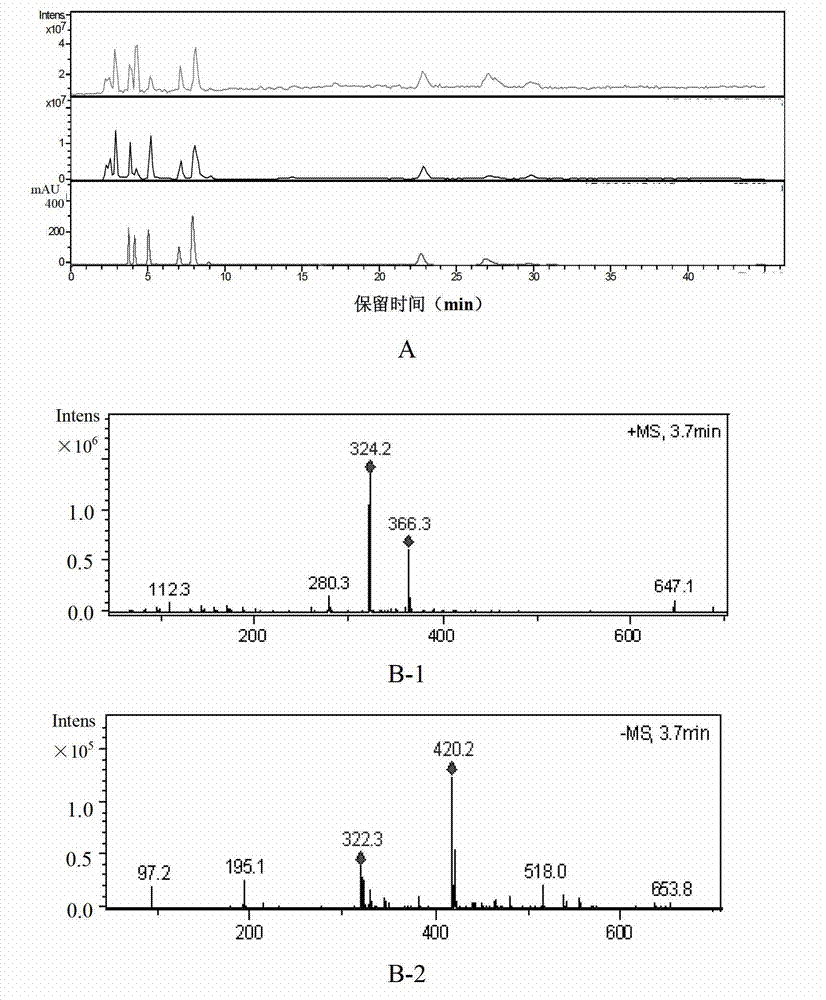
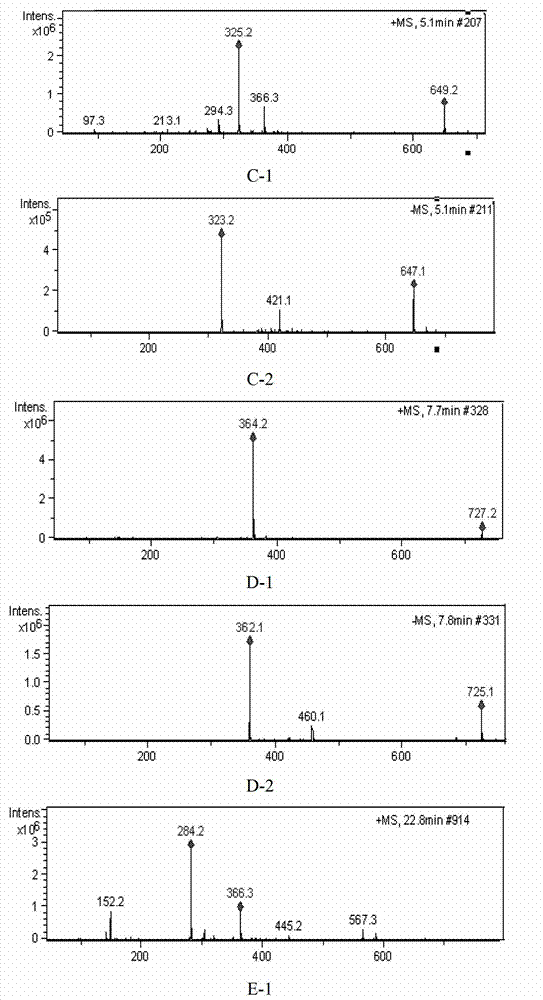
[0056] Accurately weigh 10 batches of ribonucleic acid II powder for injection, 10 mg each, into a 5ml measuring bottle, add 2 mg of nuclease, dissolve in water and set the volume to the mark, hydrolyze in a water bath at 60°C for 1 hour, let cool, make up to the mark with water, and shake well , filtered (0.45μm), and the filtrate was taken to obtain 10 batches of ribonucleic acid II for injection test solution.
[0057] (2) Preparation of reference solution
[0058] Accurately weigh appropriate amounts of cytosine nucleotides, uracil nucleotides, guanosine nucleotides, guanosine, and adenosine, respect...
Embodiment 2
[0154] Embodiment 2, the investigation of the methodology of high performance liquid chromatography detection ribonucleic acid II for injection
[0155] In the present embodiment, the preparation of need testing solution, the preparation of reference substance solution and chromatographic conditions are as follows:
[0156] a) Preparation of the test solution
[0157] Precisely weigh 10mg of ribonucleic acid II powder for injection and place it in a 5ml measuring bottle, add 2mg of nuclease, add water to dissolve and set the volume to the mark, hydrolyze in a water bath at 60°C for 1 hour, let it cool, make up to the mark with water, shake well, and filter (0.45μm), take the continued filtrate, that is.
[0158] b) Preparation of reference solution
[0159] Accurately weigh an appropriate amount of the reference substance, respectively, add water to make a reference substance solution containing 1mg per 1ml, and obtain.
[0160] c) Chromatographic conditions
[0161] Ameri...
Embodiment 3
[0187] Embodiment 3, the application of high performance liquid chromatography detection ribonucleic acid II for injection
[0188] Based on the above-mentioned Example 1 and Example 2, in this example, high-performance liquid chromatography was used to detect 10 batches of ribonucleic acid II for injection, and guanine nucleotide was used as a reference (that is, peak 4 was the reference peak, denoted as S). Calculate the relative peak area and relative retention time of each common peak. In addition, the similarity of 10 batches of ribonucleic acid II for injection was calculated with the software "Similarity Evaluation System of Chromatographic Fingerprint of Traditional Chinese Medicine" (Chinese Medicine Chromatographic Fingerprint Similarity Evaluation System (version 2.0)).
[0189] Wherein, the preparation of need testing solution, the preparation of reference substance solution and chromatographic conditions are as follows:
[0190] a) Preparation of the test solutio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com